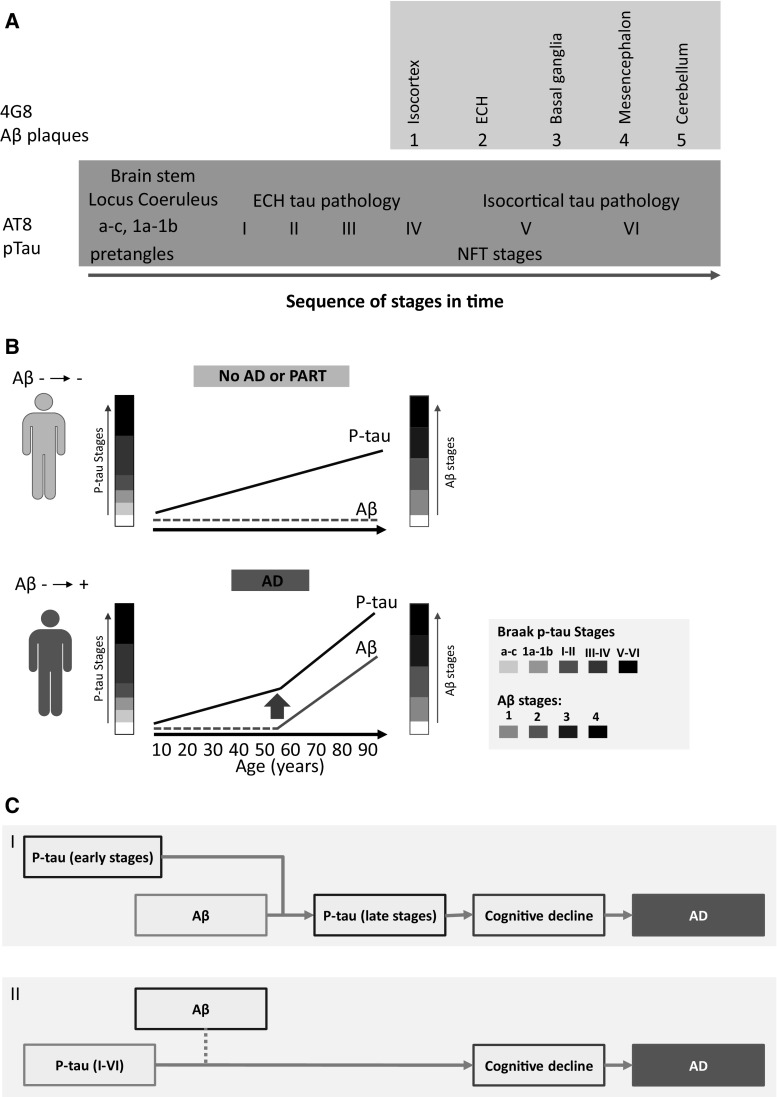
Fig. 3.
a Diagram illustrating the development of amyloid β (pink box) and p-tau (blue box) pathology (adapted from Duyckaerts et al. [33]). Post-mortem immunohistochemistry evidence of AT8-immunoreactive p-tau material, pretangles and NFT, appears at younger age than 4G8-immunoreactive amyloid β deposits [27]. b Diagram illustrating two hypothetical individual situations. The upper (green) individual does not develop amyloid β or Alzheimer’s disease. Crary et al. suggested diagnosing these cases as PART (primary age-related tauopathy) [32]. The lower (red) individual does develop amyloid β and AD and shows an acceleration of tauopathy after the appearance of amyloid β [39]. P-tau stages depicted are according to the Braak stages [27]; Amyloid β phases 1–4 are depicted according to the Thal phases of amyloid β plaques [40]. c Diagram illustrating the two current main hypotheses of the cause of Alzheimer’s disease. I The amyloid hypothesis, which has been the predominant framework for research in Alzheimer’s disease (AD), postulates that amyloid β peptide (Aβ) is the causative agent in AD. It is hypothesized that amyloid β depositions, or possibly amyloid β oligomers, accelerate the already ongoing benign early stages of p-tau pathology towards later stages, eventually resulting in AD [39]. II The p-tau hypothesis postulates that p-tau pathology is a continuum of stages of p-tau deposition, which starts early in life with AT8-immunoreactive pretangles and eventually causes AD. Extracellular and aggregated amyloid β depositions may only be produced under pathological conditions by nerve cells that contain abnormal tau (indicated with dotted blue line) [36]. Red-framed text-boxes indicate cause. (Color figure online)