Table 1.
Optimization of Alkynylation of Acetal 2a[a]
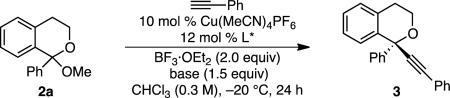
| ||||
|---|---|---|---|---|
| entry | L* | base | yield(%)[b] | ee (%)[c] |
| 1[d],[e] | L1 | iPr2NEt | 21 | 8 |
| 2[e] | L1 | iPr2NEt | 20 | 0 |
| 3 | L1 | iPr2NEt | 83 | 4 |
| 4 | L2 | iPr2NEt | 83 | 37 |
| 5 | L3 | iPr2NEt | 24 | 27 |
| 6 | L2 | DBU | 16 | 36 |
| 7 | L3 | DBU | 32 | 81 |
| 8 | L3 | MTBD | 22 | 84 |
| 9[f] | L3 | MTBD | 12 | 87 |
| 10[f],[g],[h] | L3 | MTBD | 87 | 78 |
Conditions: Acetal 2a (0.08 mmol, 1.0 equv), Cu(MeCN)4PF6 (0.008 mmol, 10 mol %), L*(0.01 mmol, 12 mol %), phenylacetylene (0.096 mmol, 1.2 equiv), BF3·Et2O (0.16 mmol, 2.0 equv), base (0.12 mmol, 1.5 equv), CHCl3 (0.3M), −20 °C, 24 h, unless otherwise noted.
Determined by 1H NMR analysis using 1,3,5-trimethoxybenzene as internal standard.
Determined by HPLC analysis using a chiral stationary phase.
TMSOTf (1.2 equiv) replaced BF3·-Et2O.
Et2O instead of CHCl3.
CuSPh instead of Cu(MeCN)4PF6.
MTBD (1.55 equiv), CHCl3 (0.15 M).
4 °C.
