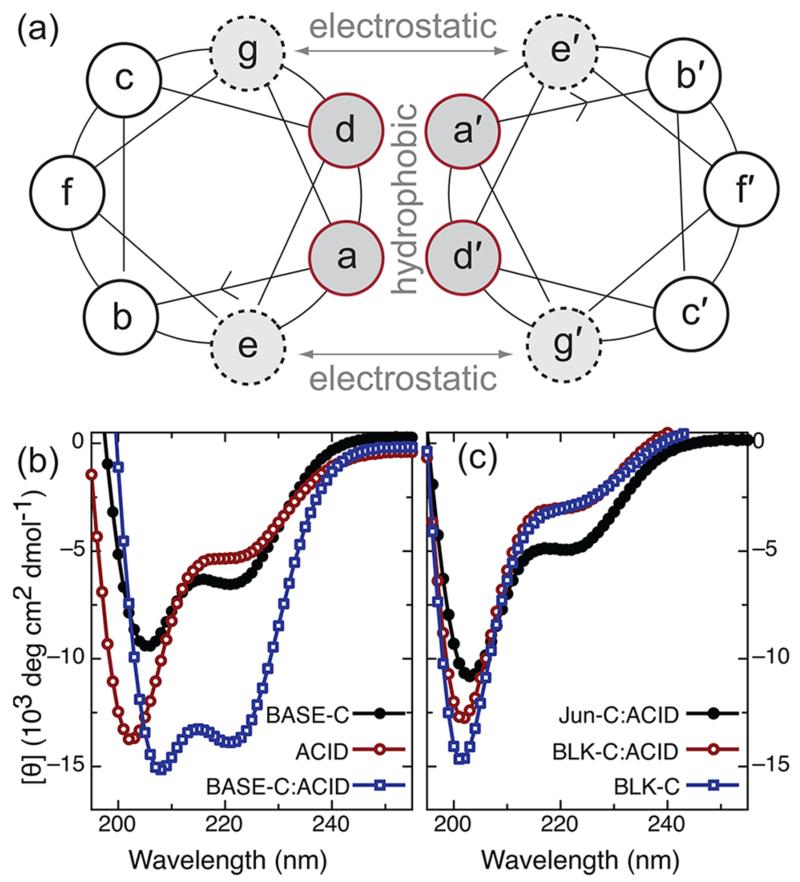
Figure 1.
(a) Helical wheel representation of the heptad repeat associated with a coiled-coil dimer. Dimer stability and specificity is regulated by a combination of hydrophobic interactions that occur between residues a and d and electrostatic interactions between amino acids at positions g and e. (b) Circular dichroism (CD) spectra for the individual peptides BASE-C and ACID and for the BASE-C:ACID heterodimer. (c) CD spectra for the random-coil peptide BLK-C and for solutions of BLK-C mixed with ACID and Jun-C mixed with ACID. For all CD measurements, peptides were dissolved in 0.1 M sodium phosphate at pH 7 at 0.25 mg/mL. For experiments involving mixed peptide solutions (i.e., BASE-C:ACID, BLK-C:ACID, and Jun-C:ACID), the solutions were allowed to incubate for 30 min at room temperature before the measurement was performed.