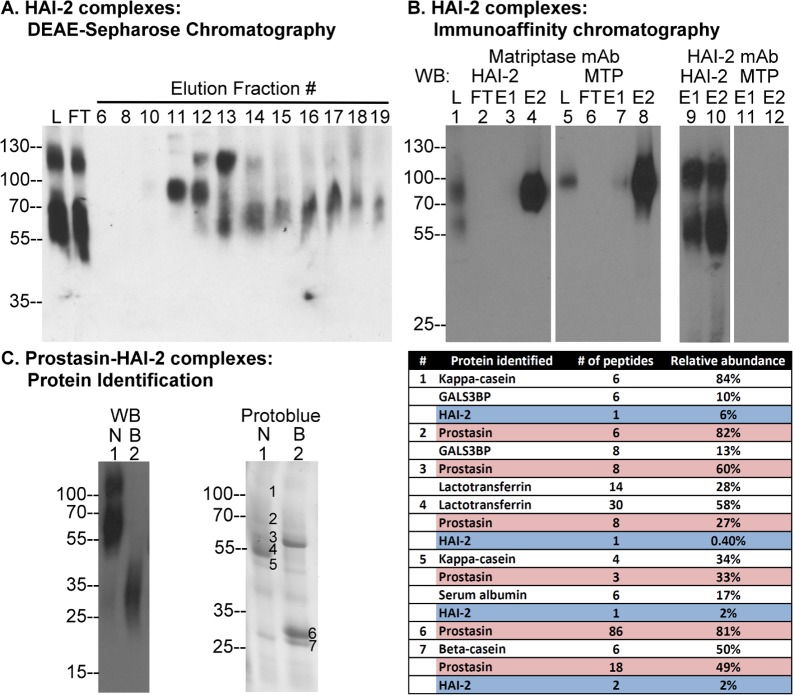
Fig 3. Purification and identification of HAI-2 complexes in human milk.
(A) The unbound fraction from the CM-Sepharose column was subjected to DEAE-Sepharose chromatography and the bound proteins eluted by a gradient of sodium chloride. The loaded fraction (lane L), flow-through fraction (FT), and eluted fractions 6–19, were analyzed by Western blot for HAI-2 species. (B) The DEAE fractions, containing HAI-2 species, were pooled and subjected to immunoaffinity chromatography using matriptase mAb 21-9-Sepharose followed by HAI-2 mAb DC16-Sepharose. The DEAE fraction (L), flow-through (FT), and two eluted fractions (E1 and E2) from the matriptase mAb-Sepharose were analyzed by Western blot (WB) for HAI-2 species (lanes 1–4) and matriptase (MTP) species (lanes 5–8). The eluted fractions (E1 and E2) from the HAI-2 mAb-Sepharose were analyzed by Western blot for HAI-2 species (lanes 9 and 10) and matriptase (MTP) species (lanes 11 and 12). (C) The HAI-2 species purified by immunoaffinity chromatography were analyzed by Western blot (WB) for HAI-2 species and by staining with Protoblue for the protein profile under non-boiled (N) and boiled (B) conditions. Seven protein bands, as indicated, were sliced from the gel for protein identification. The proteins, the number of peptides, and the relative abundance identified from the 7 gel bands are summarized in the table on the right.