Fig. 6. Favorable π–quaternary ammonium cation interactions with w-w H-bond pairings.
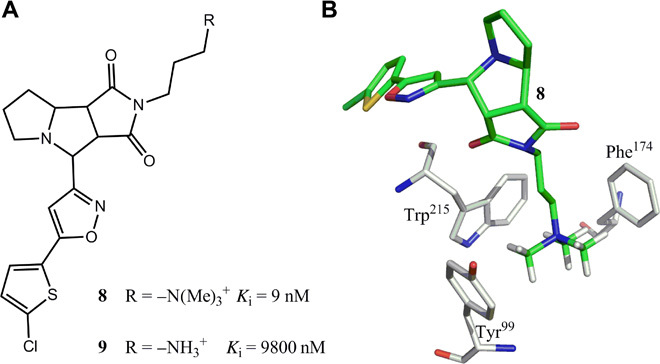
(A and B) Structures of two factor Xa antagonists 8 and 9. Antagonist 8 is ~1100-fold more active than 9 because of the w-w pairing interactions between the hydrophobic aromatic rings of factor Xa and polarized CH groups [–N(Me)3+] shown in (B) (Protein Data Bank: 2JKH).
