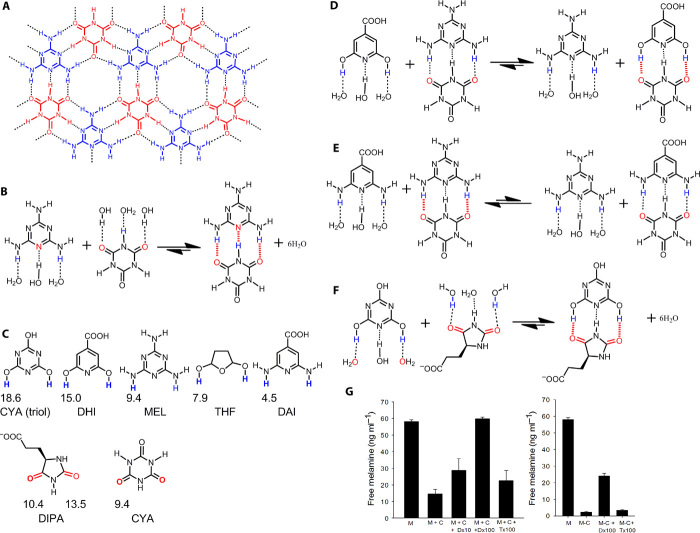
Fig. 7. Pathogenic s-s H-bond pairings in melamine toxicity.
(A) Structural analysis of melamine (MEL, blue)–cyanuric acid (CYA, red) interactions demonstrates an extensive H-bonding network (dashed lines) that promotes the formation of toxic insoluble crystals. (B and C) The MEL and CYA complex forms s-s H-bond pairings (fig. S7), with H-bonding capabilities of MEL and CYA and of inhibitors 2,6-dihydroxyisonicotinic acid (DHI), 3-(2,5-dioxo-4-imidazolidinyl)propanoic acid (DIPA), and tetrahydrofurandiol (THF) shown in (C). (D and E) Although DHI (D) and 2,6-diaminoisonicotinic acid (DAI) (E) are structurally similar, the hydrogen atoms of DHI have a much stronger H-bonding capability (C). (G) (left) We used this calculation of H-bonding capability to demonstrate that DHI preferentially forms a complex with CYA when compared with DAI or MEL (F). Statistically significant inhibition of MEL-CYA complex formation was also demonstrated with DIPA, which forms s-s H-bond pairings with the triol tautomer of CYA. The triol tautomer predominates in solution because of its aromatic character. DIPA also dissolved MEL-CYA crystal in solution (whereas THF did not) (G; right), illustrating how the s-s/w-w H-bond pairing principle may be applied to lead optimization in diseases caused by low solubility (error bars show SEM; P < 0.05 was considered significant using t test or Mann-Whitney U test for ranks).