Fig. 8. Lead optimization of the C. difficile toxin inhibitor InsP6 using the s-s/w-w H-bond pairing principle.
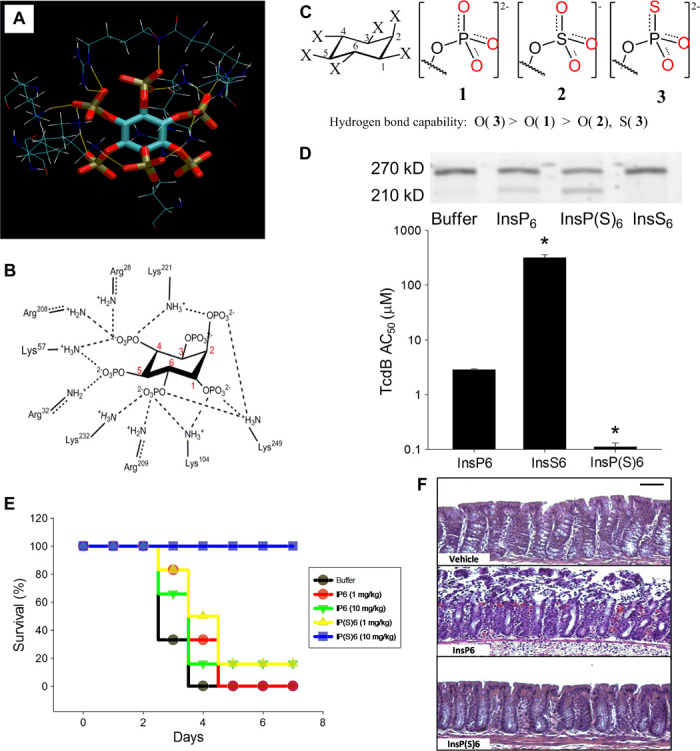
(A and B) H-bond interactions between InsP6 and allosteric binding site residues on TcdB based on the crystal structure 3PA8. (C) Structures of InsP6, its derivative InsP(S)6, and the structural analog InsS6 and the relative H-bonding capability of the oxygen atoms. (D) (Top) TcdB autocleavage induced by 100 nM InsP6, InsP(S)6, or InsS6 shows intact unprocessed (270 kD) and processed toxin cleavage products (205 kD). Processed toxin is inactive as the virulent glucosyltransferase domain fails to enter the target cell. (Bottom) InsP6 binding affinity for TcdB as measured by its self-cleavage activity in vitro (half-maximum activation constant, AC50, in micromole per liter). Increasing the H-bonding capability of the oxygen atoms in InsP(S)6 enhances the AC50 by 26-fold, whereas decreasing the H-bonding capability in InsS6 leads to a 110-fold reduction of AC50 (*P < 0.05 compared with InsP6). (E) Kaplan-Meier survival plots of C57BL/6 mice inoculated intragastrically with 103 C. difficile VPI 10463 spores and with InsP6 or InsP(S)6 (1 or 10 mg kg−1 day−1; n = 5 per group; survival at day 4; P < 0.05, analysis of variance on ranks). (F) Histopathology showing that oral InsP(S)6 is protective for colonic mucosa when administered at 10 mg.kg−1 day−1 (scale bar, 50 μm).
