Fig. 2. Role of TLR9 in macrophage activation.
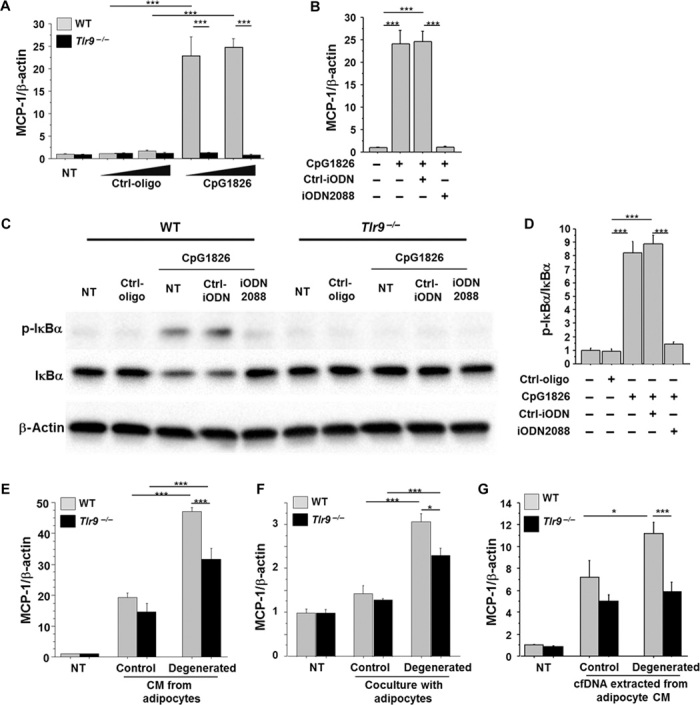
(A) CpG-ODN1826 (CpG1826) (0.1 to 1.0 μM), a TLR9-specific ligand, increased the expression of MCP-1 in peritoneal macrophages obtained from WT mice, but not in macrophages obtained from Tlr9−/− mice (n = 6). NT, non-treatment. (B) iODN2088 (0.1 μM), a specific antagonist of TLR9, inhibited the MCP-1 expression induced by CpG1826 (0.1 μM) in WT macrophages (n = 6). (C and D) Ligation of CpG1826 (0.1 μM) to TLR9 activated the NF-κB pathways determined by the phosphorylation of IκBα in WT macrophages, which was abolished by iODN2088. Neither CpG nor iODN2088 influenced the phosphorylation of IκBα in Tlr9−/− macrophages. Representative figure of Western blot analysis of IκBα phosphorylation (C) and the result of the quantification of phosphorylated IκBα normalized to the corresponding signal for total IκBα by densitometry (D) are shown (n = 4). (E) CM from control 3T3-L1 adipocytes increased MCP-1 expression in WT and Tlr9−/− macrophages. CM from degenerated adipocytes further promoted MCP-1 expression in WT macrophages, although this response was attenuated in Tlr9−/− macrophages (n = 5). After a 24-hour pretreatment with or without TNF-α, adipocytes were cultured in a starvation medium without TNF-α for another 24 hours. Culture media were then collected as CM of degenerated or control adipocytes, respectively, and used in the experiments. (F) Coculture of macrophages and 3T3-L1 adipocytes using a Transwell membrane slightly increased MCP-1 expression in WT and Tlr9−/− macrophages. Coculture with degenerated adipocytes increased MCP-1 expression in WT macrophages more efficiently, although this response was attenuated in Tlr9−/− macrophages (n = 6). (G) cfDNA extracted from degenerated adipocyte CM promoted MCP-1 expression in WT macrophages, but not in Tlr9−/− macrophages (n = 6 to 8). cfDNA extracted from 130 μl of CM was used to stimulate each well. CM from degenerated adipocytes were collected as shown in (E). *P < 0.05 and ***P < 0.001. All values are means ± SEM.
