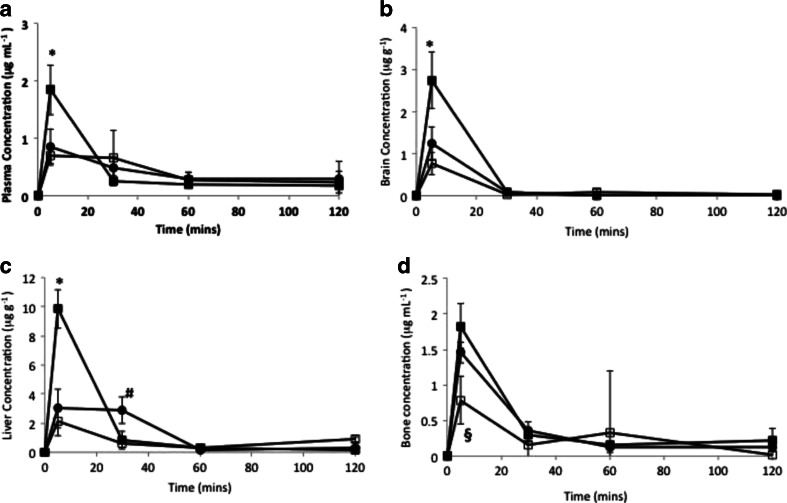
Fig. 4.
The biodistribution of MET - lomustine nanomedicine formulations: a = plasma, b = brain, c = liver, d = bone (including bone marrow), ■ = MET – lomustine (13 mg kg−1), □ MET - lomustine (6.5 mg kg−1), ● = ethanolic lomustine (6.5 mg kg−1). The MET formulation improves the delivery of lomustine to the brain by 2 fold by enabling a higher dose to be administered. Additionally, when compared to an ethanolic formulation of the drug at a dose of 6.5 mg kg−1, the MET nanoparticles reduces the exposure (AUC0 – 4h) of the bone marrow and liver to lomustine by 25 and 38% respectively, * = significant difference between the high dose MET formulation (13 mg kg−1) and all other formulations (p < 0.05), # = significant difference between ethanolic formulation and both MET formulations (p < 0.05), § = significant difference between low dose MET formulation (6.5 mg kg−1) and all other formulations (p < 0.05).