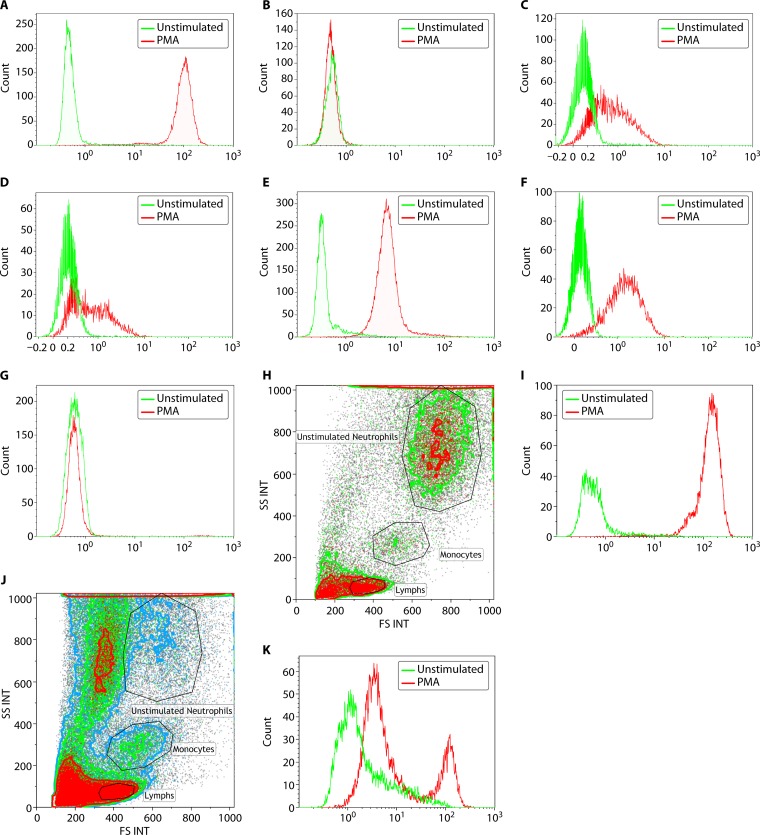
FIG 4.
Assessment of neutrophil oxidative burst using DHR flow cytometry. NADPH oxidase, which produces the respiratory burst in neutrophils, can be assessed following in vitro stimulation with PMA. The oxidation of DHR to fluorescent rhodamine is measured by flow cytometry. The unstimulated fluorescence is represented by the green histogram, while the PMA-stimulated oxidative burst in neutrophils is represented by the red histogram. Both percent-positive neutrophils and mean fluorescence intensity (MFI) are captured for diagnostic interpretation. (A) A normal neutrophil oxidative burst is demonstrated by a complete shift in the stimulated signal. (B) Data on the assessment of neutrophil oxidative burst using DHR flow cytometry in a patient with X-linked CGD. In a patient with a mutation in the CYBB gene (encoding gp91phox protein), there is no evidence of a normal neutrophil oxidative burst, and the unstimulated (green) and stimulated (red) histograms overlap completely. (C and D) Assessment of neutrophil oxidative burst using DHR flow cytometry in a patient with autosomal recessive CGD due to NCF1 and NCF2 mutations, respectively. Patients with autosomal recessive CGD, due to defects in p47phox, have a different pattern of neutrophil oxidative burst, with a proportion of neutrophils negative (stimulated histogram overlapping with unstimulated) and the remaining neutrophils showing significantly reduced fluorescence. Patients with defects in p67phox have a similar pattern of neutrophil oxidative burst to those with NCF1 mutations, with a proportion of neutrophils negative (stimulated histogram overlapping with unstimulated) and the remaining neutrophils showing significantly reduced fluorescence. (E) Assessment of neutrophil oxidative burst using DHR flow cytometry in a patient with complete myeloperoxidase deficiency (cMPO). Patients with cMPO having mutations in the MPO gene may demonstrate a variable pattern of DHR fluorescence, ranging from partially positive to completely absent. In this example, there is complete shift of the neutrophils, indicative of the majority of neutrophils showing DHR fluorescence, but there is an overall significant reduction in the mean fluorescence intensity. This would suggest partially reduced but not completely absent neutrophil oxidative burst in this patient example. (F) Assessment of neutrophil oxidative burst using DHR flow cytometry in a male patient with atypical X-linked CGD. Neutrophil oxidative burst may be preserved in some patients with mutations in the CYBB gene, leading to an atypical clinical phenotype as well as flow cytometric pattern of DHR fluorescence. The pattern is similar to that seen with NCF4 gene mutations (p40phox) and the form of complete MPO deficiency seen in panel E. This is a young male patient with one episode of Burkholderia pneumonia with no other manifestations of CGD who had a missense mutation (c.1061A>G; p.H354R) in the CYBB gene, which has been previously reported to be associated with decreased but not absent NADPH oxidase activity in neutrophils. (G) Assessment of neutrophil oxidative burst using DHR flow cytometry in a female patient with extreme skewing of lyonization, resulting in a phenotype of X-linked CGD. Neutrophil oxidative burst assessment by DHR fluorescence in female carriers of X-linked CGD characteristically shows two populations consistent with the presence of mutant and normal alleles. However, if there is skewing of lyonization, the proportion of neutrophils that are negative for DHR fluorescence can increase. This is an example of an elderly female patient with a history of being a carrier for XL-CGD and who has an affected male offspring demonstrating age-related extreme skewing of lyonization with a DHR flow pattern similar to that seen in a male patient with XL-CGD. (H and I) Side scatter (SSC) and forward scatter (FSC) separation of neutrophils, monocytes, and lymphocytes in whole blood. The DHR flow analysis is performed on neutrophils. (H) A transported sample received within validated stability under optimal conditions with abundant viable neutrophils. INT, integral. (I) DHR fluorescence for PMA-stimulated neutrophils in this sample, indicating a normal and robust result. (J and K) Another transported sample, also received within validated stability, but under suboptimal conditions. (J) There is significant neutrophil cell death observed with reduced viable neutrophils. (K) DHR fluorescence from PMA-stimulated neutrophils of the same sample with high background in unstimulated control (green histogram) and two peaks (red histogram), indicating poor sample quality.