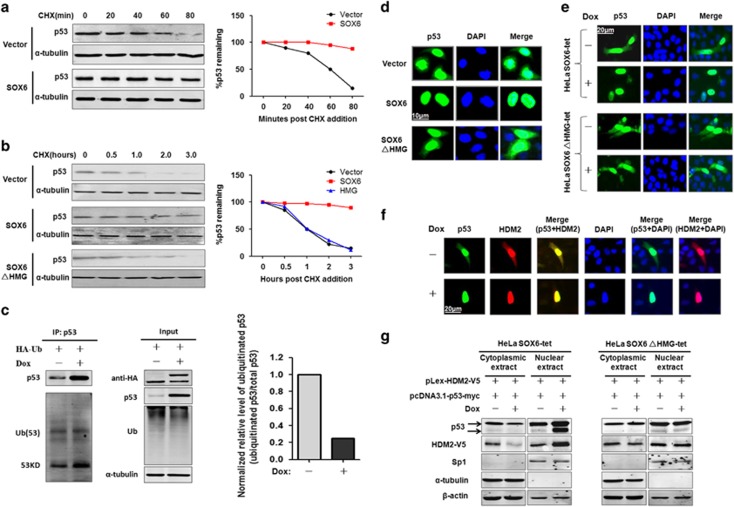
Figure 4.
SOX6 increases the stability of p53 protein by inhibiting the nuclear export and ubiquitination of p53. Turnover assay of p53 protein in HeLa cells (a) and SMMC7721 cells (b) that had been transfected with SOX6, SOX6ΔHMG or control expression vector with cycloheximide (CHX) (100 μg/ml) treatment at 48 h post transfection for the indicated times. (c) Immunoprecipitation and western blot assays were conducted to determine the effect of SOX6 on p53-ubiquitination/HDM2 activity in HeLa SOX6-tet cells transfected with a ubiquitination expression vector and treated with or without Dox (2 μg/ml) for 48 h. (d) Immunocytofluorescence staining of p53 in HeLa cells that had been co-transfected with pcDNA3.1-p53-myc and pLex-HA-SOX6, pLex-HA-SOX6ΔHMG or control expression vector. Nuclei (blue) are stained with 4'-6-diamidino-2-phenylindole (DAPI). (e) Immunocytofluorescence staining of p53 in HeLa SOX6-tet cells that had been transfected with pcDNA3.1-p53-myc and treated with or without Dox (2 μg/ml) for 48 h. (f) Immunocytofluorescence staining of p53 and HDM2 in the same field of HeLa SOX6-tet cells that had been co-transfected with pcDNA3.1-p53-myc and pLex-HDM2-V5 plasmids. Colocalization of p53 and HDM2 was analyzed by merging the images of p53 and HDM2 in the same field. (g) Western blot was performed to detect the cytoplasmic or nuclear protein levels of p53 and HDM2 in HeLa SOX6-tet and SOX6ΔHMG-tet cells that had been co-transfected with pcDNA3.1-p53-myc and pLex-HDM2-V5 plasmids. Cytoplasmic and nuclear proteins were extracted at 48 h of transfection, and purity of the nuclear and cytoplasmic extracts was indicated by SP1 and α-tubulin, respectively.