Figure 2. Biochemical analysis of direct interactions of purified H. pylori TlpD and AcnB or TlpD and KatA proteins using biolayer interferometry.
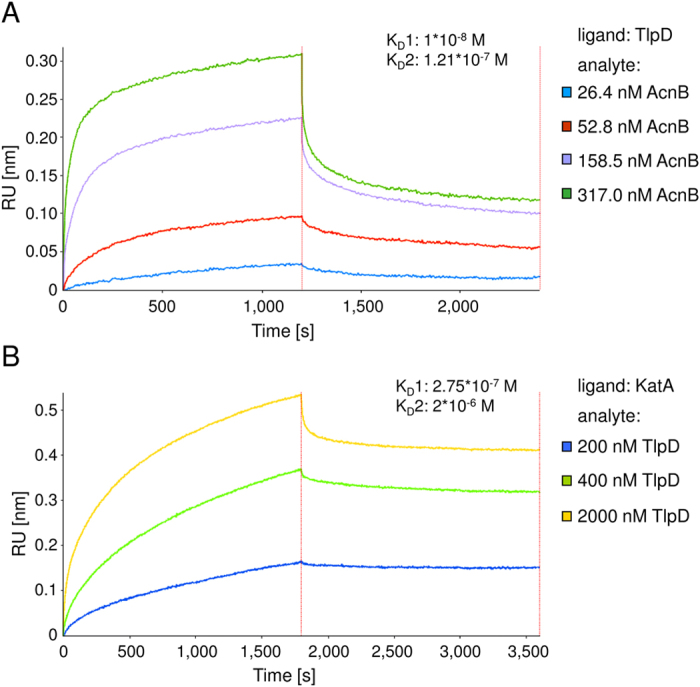
Recombinantly purified TlpD was tested for direct interactions with purified H. pylori AcnB or KatA using biolayer interferometry. While one purified protein (ligand) was coupled to the sensor surface via a hexa-histidine tag or free amine groups (see Supplementary Methods), the second protein (analyte) was applied as a solute in assay buffer. Sensors were dipped into different analyte solutions for 1,200 sec (A) or 1,800 sec (B), before dissociation was monitored in assay buffer. (A) TlpD-AcnB interaction: ligand Hisx6-TlpD (purified from E. coli) was immobilised to the sensor surface, while analyte H. pylori AcnB (AcnB-V5, purified from H. pylori) was applied in assay buffer (Methods and Supplementary Methods) at four different concentrations (26.4 nM, 52.8 nM, 158.5 nM and 317.0 nM). (B) TlpD-KatA interaction: immobilised H. pylori KatA (recombinant, tag-free, purified from E. coli) was coupled on the sensor surface via free amine groups, while analyte TlpD-V5 (purified from H. pylori) was supplied diluted in assay buffer at 200 nM, 400 nM and 2,000 nM. Values derived from a ligand-coupled sensor that was dipped in assay buffer only over the full time course of the interaction assay were subtracted as the baseline from the interaction curves.
