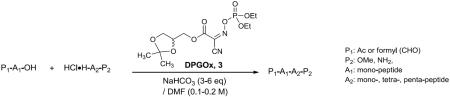
Table 2.
| entry |
N-protected α-amino acid |
C-protected α-amino acid |
conditionsa | time (h) |
product | yield (%) |
de (%) |
|---|---|---|---|---|---|---|---|
| [1+1] | |||||||
| 1 | Ac-L-Phe-OH | HCl•H-L-Ala-OMe | A | 2 | Ac-L-Phe-L-Ala-OMe | 85 | >99b |
| 2 | Ac-L-Tyr-OH | HCl•H-L-Ala-OMe | A | 2 | Ac-L-Tyr-L-Ala-OMe | 75 | >99b |
| 3 | Ac-L-Tyr-OH | HCl•H-L-Ala-NH2 | B | 2 | Ac-L-Tyr-L-Ala-OMe | 95 | >92c |
| 4 | Ac-L-Ser-OH | HCl•H-L-Ala-OMe | A | 2 | Ac-L-Ser-L-Ala-OMe | 85 | >99b |
| 5 | CHO-L-Leu-OH | HCl•H-L-Ala-OMe | A | 3 | CHO-L-Leu-L-Ala-OMe | 70 | >99b |
| 6 | CHO-L-Leu-OH | HCl•H-L-Ala-OMe | B | 3 | CHO-L-Leu-L-Ala-OMe | 95 | >99b |
| 7 | CHO-L-Phe-OH | HCl•H-L-Ala-OMe | A | 3 | CHO-L-Phe-L-Ala-OMe | 73 | >99b |
| 8 | CHO-L-Phe-OH | HCl•H-L-Ala-OMe | B | 3 | CHO-L-Phe-L-Ala-OMe | 95 | >99b |
| [4+1] | |||||||
| 9 | Ac-L-Lys(Z)-OH | HCl•H-L-Ala-L-Lys(Z)-L-Lys(Z)-L-Phe-OMe | A | 2 | Ac-L-Lys(Z)-L-Ala-L-Lys(Z)-Lys(Z)-L-Phe-OMe | 80 | >92c |
| 10 | CHO-L-Lys(Z)-OH | HCl•H-L-Ala-L-Lys(Z)-L-Lys(Z)-L-Phe-OMe | A | 2 | CHO-L-Lys(Z)-L-Ala-L-Lys(Z)-L-Lys(Z)-L-Phe-OMe | 81 | >92c |
| [5+1] | |||||||
| 11 | Ac-L-Lys(Z)-OH | HCl•H-L-Lys(Z)-L-Ala-L-Lys(Z)-L-Lys(Z)-L-Phe-OMe | A | 3 | Ac-L-Lys(Z)-L-Lys(Z)-L-Ala-L-Lys(Z)-L-Lys(Z)-L-Phe-OMe | 80 | >92c |
| 12 | CHO-L-Lys(Z)-OH | HCl•H-L-Lys(Z)-L-Ala-L-Lys(Z)-L-Lys(Z)-L-Phe-OMe | A | 3 | CHO-L-Lys(Z)-L-Lys(Z)-L-Ala-L-Lys(Z)-L-Lys(Z)-L-Phe-OMe | 80 | >92c |
A: N-acyl-α-amino acid (1.5 equiv), C-protected α-amino acid (1.0 eqiv), 3 (1.5–2.0 equiv), NaHCO3 (3 equiv), DMF (0.2 M); B: N-acyl-α-amino acid (3.0 equiv), C-protected α-amino acid (1.0 eqiv), 3 (3.0 equiv), NaHCO3 (3 equiv), DMF (0.2 M);
de was determined by HPLC;
de was determined by 1H-NMR analysis;