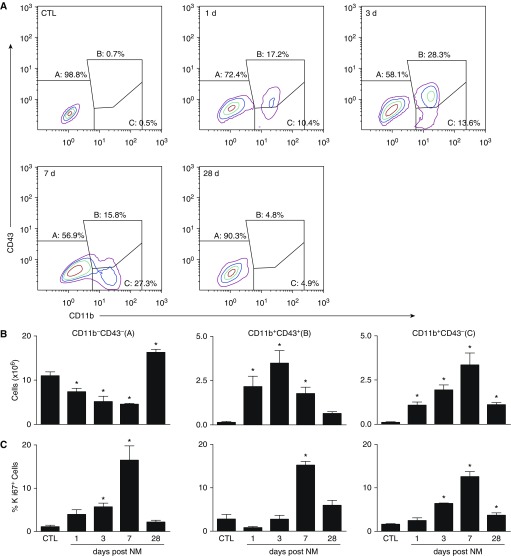
Figure 4.
Flow cytometric analysis of lung macrophages. Macrophages, isolated 1, 3, 7, and 28 days after exposure of rats to PBS CTL or NM, were incubated with anti–rat-FcRII/III antibody (1 μl/106 cells) for 10 minutes at 4°C, followed by incubation (30 min) with AlexaFluor 488 (AF488) anti-CD11b and AF647 anti-CD43 antibodies or appropriate isotype controls, and then with eFluor780 viability dye (30 min). Cells were then analyzed by flow cytometry. Macrophages were identified based on forward and side scatter followed by doublet discrimination of live cells. (A) Representative contour plot showing resident alveolar macrophages (A; CD11b−CD43−), infiltrating immature macrophages (B; CD11b+CD43+), and infiltrating mature macrophages (C; CD11b+CD43−). (B) Numbers of CD11b−CD43−, CD11b+CD43+, and CD11b+CD43− cells were calculated from the percentage of positive macrophages relative to the total number of lung cells recovered. (C) Following labeling with CD11b, CD43, and viability dye, cells were incubated for 30 minutes in Fixation/Permeabilization buffer (eBioscience, San Diego, CA), followed by 30 minutes of incubation with eFluor450 Ki67 antibody. Cells were then fixed in 2% paraformahaldehyde and analyzed by flow cytometry. Error bars, mean ± SE (n = 3–5 rats). *Significantly different (P ≤ 0.05) from CTL.