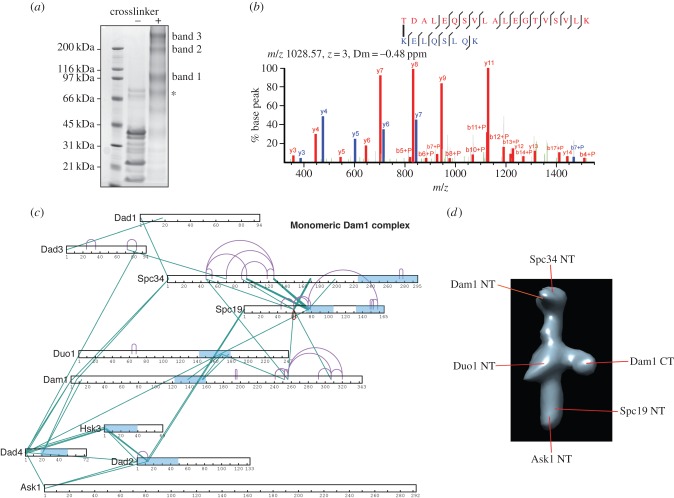
Figure 1.
Isolation and architecture of monomeric Dam1 complex by cross-linking. (a) SDS–PAGE analysis of the Dam1 complex cross-linked with BS3. Cross-linked Dam1 complex was excised and analysed by mass spectrometry. Asterisk denotes that excluded from our analysis. (b) Fragmentation spectrum of a cross-linked peptide that includes a link between Lys-13 in Dad2 and Thr-2 in Spc19. Peaks supporting TDALEQSVLALEGTVSVLK are annotated in red, and those supporting KELQSLQK are annotated in blue. (c) Intersubunit and intramolecular cross-link maps for the monomeric Dam1 complex. Intramolecular and intermolecular cross-links are coloured purple and green, respectively. Predicted coiled coil regions are in light blue. (d) Representative EM map of the monomeric Dam1 complex (modified from EMDB: 1972), highlighting the reported positions of subunit termini in previous studies.