Abstract
Damage to the superior and/or inferior parietal lobules (SPL, IPL) (Sirigu et al., 1996) or cerebellum (Grealy and Lee, 2011) can selectively disrupt motor imagery, motivating the hypothesis that these regions participate in predictive (i.e., feedforward) control. If so, then the SPL, IPL, and cerebellum should show greater activity as the demands on feedforward control increase from visually-guided execution (closed-loop) to execution without visual feedback (open-loop) to motor imagery. Using fMRI and a Fitts’ reciprocal aiming task with tools directed at targets in far space, we found that the SPL and cerebellum exhibited greater activity during closed-loop control. Conversely, open-loop and imagery conditions were associated with increased activity within the IPL and prefrontal areas. These results are consistent with a superior-to-inferior gradient in the representation of feedback-to-feedforward control within the posterior parietal cortex. Additionally, the anterior SPL displayed greater activity when aiming movements were performed with a stick vs. laser pointer. This may suggest that it is involved in the remapping of far into near (reachable) space (Maravita and Iriki, 2004), or in distalization of the end-effector from hand to stick (Arbib et al., 2009).
Keywords: Tool use, Motor imagery, Visually guided movement, Sensorimotor control, Posterior parietal cortex, Cerebellum
Introduction
It is generally accepted that most movements, including the use of tools, involve both feedback and feedforward (predictive) control, the latter relying on internal models that generate predictions of the sensory consequences of motor commands (Ito, 1984; Kawato, 1999; Wolpert and Flanagan, 2001). The contributions of these respective controllers are thought to vary according to prevailing task demands in an attempt to optimize performance (Kording and Wolpert, 2006; Todorov, 2004). Isolating the neural mechanisms that implement these processes has, however, proven to be quite challenging, and the superior parietal lobule (SPL), inferior parietal lobule (IPL), and cerebellum have been implicated in both the feedback and feedforward control of movements (Blakemore and Sirigu, 2003; Desmurget and Grafton, 2000; Frey et al., 2011; Imamizu et al., 2000; Wolpert et al., 1998a; Wolpert et al., 1998b), including those with tools (Higuchi et al., 2007).
One approach to this challenge has involved the use of motor imagery—the mental rehearsal of movements in the absence of execution and thus sensory feedback. Given that feedback is absent, imagery might therefore involve predicting what the sensory consequences would be for inhibited motor commands. This has led to claims that motor imagery might provide a vehicle for investigating mechanisms of feedforward control (Frey, 2010; Grush, 2004; Shadmehr and Krakauer, 2008). In a seminal study, Sirigu et al. (1996) found that, in contrast to overt movements with a stylus, imagined movement times in a reciprocal aiming task did not obey Fitts’ Law1 for patients with SPL and/or IPL lesions. They concluded that these posterior parietal areas must be important for the mental representation of movements, and more precisely for accurate prediction of the time required to complete them. Subsequently, these findings have been interpreted as evidence for the involvement of posterior parietal cortex in feedforward control (Shadmehr and Krakauer, 2008; Wolpert and Flanagan, 2001). More recent findings have shown that injury to the cerebellum can also cause a dissociation between imagined and executed movements (Gonzalez et al., 2005; Grealy and Lee, 2011), suggesting that it too may be involved in feedforward control.
There are, however, at least two major limitations to these claims. First, it remains unknown whether these findings are peculiar to patients with brain damage, or whether they generalize to the functional architecture of the healthy brain. Second, the hypothesis that motor imagery is a reflection of isolated feedforward control is debatable. Sensorimotor control involves dynamic interaction between feedforward and feedback mechanisms while imagery completely lacks both descending efferent (motor) and afferent (visual and proprioceptive) signals. There is also some evidence that imagery tasks can be solved using alternative strategies (Rodriguez et al., 2008; Viswanathan et al., 2012).
In an attempt to clarify these two issues, we undertook an investigation using a variant of the classic Fitts’ (1954) reciprocal aiming paradigm while recording brain activity with fMRI in a sample of healthy adults. In addition to the closed-loop execution and motor imagery conditions of the preceding patient-based investigations, we included an intermediate condition in which reciprocal aiming was performed with full vision of the targets, but without visual feedback of hand or tool movements (open-loop). We reasoned that the selective removal of visual feedback during open-loop execution would precipitate a greater reliance on predictive feedforward control than the visually-guided execution (closed-loop) condition, while still allowing proprioceptive feedback. This should minimize the likelihood that alternative strategies would be employed to solve the task, a potential risk for the imagery condition.
On the basis of previous behavioral findings (Macuga et al., 2012), we predicted that movement durations for all three conditions would conform to Fitts’ Law, but that slopes for participants’ estimates of movement times in the imagery condition would be smaller, reflecting a reduced influence of task difficulty (ID) on performance. As in earlier neuroimaging studies of visually-guided aiming, we expected that the closed-loop condition would involve the SPL, IPL, and cerebellum in addition to other regions associated with sensorimotor control (Seidler et al., 2004; Winstein et al., 1997). For the open-loop condition, we predicted greater reliance on feedforward control and an associated increase in activity within the posterior parietal cortex (SPL and IPL) and the cerebellum. Finally, to the extent that motor imagery relies on feedforward control, then these same regions should show further increases in activity during the motor imagery condition where feedback control is not an option.
A secondary aim of this study was to assess the effects of tool use on the representation of far space in the parietal cortex. When a stick is used to extend the arm’s reach, previously far space appears to be represented as near (Iriki et al., 1996; Maravita and Iriki, 2004). This remapping does not occur when a laser pointer is used, presumably because, unlike the stick, it is ineffective at manipulating the distant environment (Berti and Frassinetti, 2000; Longo and Lourenco, 2006; Pegna et al., 2001). Recent fMRI evidence suggests that the human superior parieto-occipital cortex (SPOC) may be particularly responsive to action targets located within reach of the hand (Gallivan et al., 2009). However, SPOC responses were unchanged when a tool was used to extend reachable space. This raises the possibility that SPOC codes near, as opposed to reachable, space, the latter being contextually variable depending on the presence or absence of a tool. In an attempt to clarify this issue, targets in our reciprocal aiming task were always presented beyond reach of the hand (in far space), and participants used either a stick or laser pointer matched for torque and dynamics. If SPOC codes objects within reach (regardless of their actual locations), then it should show increased activity when performing with the stick (which can affect targets in far space) vs. the laser (which cannot affect far space). Alternatively, if SPOC codes objects within near space, then it should be equally unresponsive in both conditions.
Methods
Participants
Twenty right-handed volunteers (19–28 years,6 females) with normal or corrected-to-normal visual acuity and no reported history of neurological disease participated. Each gave written informed consent, and the local ethics committee approved the experimental protocol.
Stimuli
A customized program developed with National Instruments LabVIEW software and a PCI-1405 image acquisition board controlled stimulus presentation and synchronization with the MRI scanner. An MRI-compatible hi-res miniature CCD video camera (COPS-CG35H) was mounted at the edge of the scanner bore and captured the scene from the participant’s perspective. The video stream was then back-projected (InFocus IN20 Series projector) onto a screen located at the head of the bore and viewed by participants on a small (5″ × 2″) mirror attached to the head coil. Fig. 1 illustrates sample views of the actual MRI set-up from the participant’s perspective. Stimuli were physically placed into a slotted wooden apparatus by the experimenter. The stimuli were presented at a distance of 36″ and consisted of 8.5″ × 11″ cards marked with an 80 mm high × 24 mm wide start box, as well as a square target box to the left of the start box. The closest edge of the target box was 90 mm away and centered in relation to the start box. There were 3 different sizes of square target boxes: 20 mm, 40 mm, and 80 mm. Equidistant from the start box and target box, was a small square, which participants were asked to fixate as they performed the task. The task involved pointing, or imagined pointing, back and forth between the start and target boxes 8 times. In between trials, the experimenter switched the stimuli, according to a predetermined randomized order.
Fig. 1.
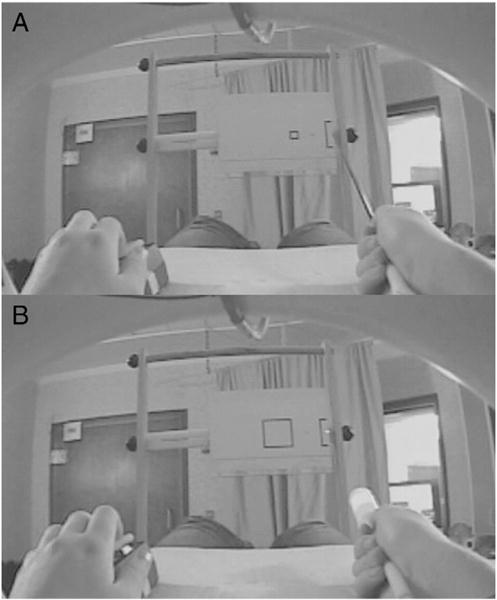
Stimulus setup from a participant’s perspective. Stimuli (8.5″ × 11″ paper sheets at a distance of 36″, attached to card stock) were placed vertically in to a wooden apparatus, attached to the scanner bed. Start box on the right was 80 mm high × 24 mm wide. Square target box 90 mm to the left of the start box was 20 mm, 40 mm, or 80 mm wide. In between the start and target boxes, was a small square, which participants were asked to fixate as they performed the task. Movement durations were recorded via left hand button release and press. A. Small (20 mm) target box and stick are shown here. B. Large (80 mm) target box and weighted laser are shown here.
We also manipulated the tool pointing task itself. The task conditions were: closed-loop—participants had access to both visual and proprioceptive information as they saw live video of the stimuli and the hand with tool as they moved it back and forth between two targets, open-loop—visual feedback of hand and tool movements was removed since participants saw a captured static image of the stimuli and hand with tool. They moved the tool back and forth as in the open-loop condition, but received NO visual feedback of their movements, and imagine—both visual and proprioceptive feedback of hand and tool movements was removed since participants saw live video of the targets and the hand with tool but mentally simulated the movements without physically moving the tool. For the imagine condition, participants were instructed to really try to feel like they were moving the tool back and forth between the targets (emphasis on kinesthetic imagery), even though they were not actually moving. They still lifted their left hands off the button and placed them back down once they had finished imagining the 8 cycles. It is important to note that the stimulus was visible in all conditions, even when we removed feedback of the hand with tool. For this condition, images were captured using the same video camera used to provide live feedback, prior to each run, with participants holding each tool in the start box for each of the three target widths (Fig. 1).
Design and procedure
Participants were given instructions and completed a 45 min training session 1 day beforehand in a mock scanner. They were told to perform the reciprocal aiming task as quickly and accurately as possible, and that landing outside either box was an error. When they made an error during the training phase, they were corrected and told to slow down if it allowed them to perform the task accurately. A left-hand button was released to indicate the start and pressed when finished, in conjunction with the right hand performing the reciprocal aiming task. The right hand held one of two tools: a stick (Fig. 1A) or a laser (Fig. 1B). The stick was a thin rigid black tube (.188″ outer diameter, .116″ inner diameter) made from high-strength lightweight carbon fiber. The laser was composed of a laser box connected to a fiber optic cable with an emitting pointer on the end. The laser box contained a green 5 mW module that was focused through a 2-axis turning mirror and mount, which allowed precise pointing onto the fiber optic connector. The pointer end had a small 8 mm fl lens which re-imaged the 50 micron fiber end to project a beam of light, which was adjusted so that the diameter on the stimulus sheet perfectly matched the diameter of the stick. Identical handles made from white Teflon were fitted to both tools. The laser was also weighted with a larger piece of white Teflon to carefully match the torque of the stick at 240 g-in. To match auditory and tactile sensations across tools, the stick did not tap against the target boxes.
The timeline of a representative block is shown in Fig. 2. Participants executed or imagined distal reciprocal aiming movements (36″ away) using a tool (stick or laser) in their right hands to targets of varying sizes (20, 40, 80 mm), while whole-brain data was acquired. Based on Fitts’ law, the 20 mm width (Fig. 1A) would result in a high ID (hardest), whereas the 80 mm width (Fig. 1B) would result in a low ID (easiest).
Fig. 2.
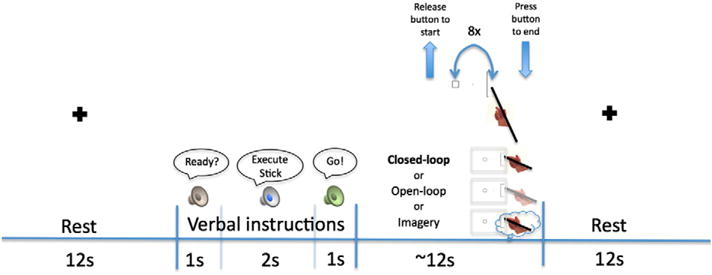
Experimental timeline shown for a representative block There were 18 conditions in a 2 (tool) × 3 (target size = ID) × 3 (task) design. Verbal presentation of the word “execute” (for task conditions both with and without visual feedback) or “imagine” distinguished task conditions followed by the word “stick” or “laser” designating tool condition just prior to each block The stick condition is shown here for illustrative purposes. After the instructional cue, participants heard the word “go” and released the button with the left hand. At the release of the button, they also began performing the task (8 full cycles of pointing back and forth between the start box and one of the target boxes–20 mm, 40 mm, or 80 mm). When finished, they pressed the button with the left hand. The button presses allowed us to record durations for both imagined and executed trials. A depiction of the three task conditions is shown: closed-loop–saw hand/tool moving via live video, open-loop–saw a captured image of hand/tool, imagine—did not physically move, just mentally simulated the movements, but saw a live video of stationary hand/tool. After each 12 s experimental block, a black cross on a white screen appeared for participants to fixate during a 12 s rest period.
Prior to each block, the stimulus appeared and participants heard “ready” and then heard recorded verbal instructions to “execute” or “imagine” with the “stick” or “laser”. The “execute” instruction applied to both closed- and open-loop conditions. When participants heard the “go” cue, they lifted their left hands off the button, performed or imagined 8 cycles of the task with their right hand plus tool, and pressed the left button to finish. Blocks of each condition lasted 12 s followed by 12 s rest (baseline) consisting of a digitally presented black fixation cross on a white screen, which was when the experimenter switched the stimulus cards. Each run (4 total) included all 18 conditions: 3 IDs (high—small target, medium—medium target, low—large target) × 3 task conditions (closed-loop, open-loop, imagine) × 2 tool types (stick, laser). The stick and laser were switched by the experimenter halfway through the run, to avoid unnecessary movement, though each set of 9 blocks was presented in a randomized order. Tool presentation was counterbalanced across runs and subjects. Time to complete (or imagine completing) 8 cycles was recorded.
Data acquisition
Scans were performed on a Siemens (Erlangen, Germany) 3 T Allegra MRI scanner. BOLD echoplanar images (EPIs) were collected using a T2*-weighted gradient echo sequence, a standard birdcage radio-frequency coil, and the following parameters: TR = 2500 ms, TE = 30 ms, flip angle = 80°, 64 × 64 voxel matrix, FoV = 220 mm, 40 contiguous axial slices acquired in interleaved order, thickness = 4.0 mm, in-plane resolution: 3.4 × 3.4 mm, and bandwidth = 2604 Hz/pixel. The initial 4 scans in each run were discarded to allow the MR signal to approach a steady state. High-res T1-weighted structural images were also acquired, using the 3D MP-RAGE pulse sequence: TR = 2500 ms, TE = 4.38 ms, TI = 1100 ms, flip angle = 8.0°, 256 × 256 voxel matrix, FoV = 256 mm, 176 contiguous axial slices, thickness = 1.0 mm, and in-plane resolution: 1.0 × 1.0 mm. DICOM image files were converted to NIFTI format using MRIConvert software (http://lcni.uoregon.edu/~jolinda/MRIConvert/).
Preprocessing
Structural and functional fMRI data were preprocessed and analyzed using fMRIB’s Software Library [FSL v.4.1.7 (http://www.fmrib.ox.ac.uk/fsl/)] (Smith et al., 2004) and involved: motion correction using MCFLIRT (Jenkinson et al., 2002), independent components analysis conducted with MELODIC to identify and remove any remaining obvious motion artifacts including strong alternation between slices, activation in non-brain areas, or a temporal spike in the timecourse (Beckmann and Smith, 2004), fieldmap-based EPI unwarping to correct for distortions due to magnetic field inhomogeneities using PRELUDE + FUGUE (Jenkinson, 2003)–with a fieldmap collected in the middle of the experiment between runs 2 and 3, non-brain matter removed using BET (Smith, 2002), data spatially smoothed using a 5 mm full-width at half-maximum Gaussian kernel, mean-based intensity normalization applied, in which each volume in the data set was scaled by the same factor, to allow for cross-sessions and cross-subjects statistics to be valid, high-pass temporal filtering with a 100 s cut-off was used to remove low-frequency artifacts, time-series statistical analysis was carried out in FEAT v.5.98 using FILM with local autocorrelation correction (Woolrich et al., 2001), delays and undershoots in the hemodynamic response accounted for by convolving the model with a double-gamma HRF function, registration to the high-resolution structural with 7 degrees of freedom and then to the standard images with 12 degrees of freedom (Montreal Neurological Institute [MNI-152] template) at a 2 × 2 × 2 voxel resolution implemented using FLIRT (Jenkinson et al., 2002; Jenkinson and Smith, 2001), and registration from high resolution structural to standard space was performed with FNIRT nonlinear registration (Andersson et al., 2007).
Behavioral analysis
Each participant’s mean movement or imagery duration was calculated for target width and sorted according to the task condition (closed-loop, open-loop, imagine) and tool type (stick, laser). The group mean durations were then plotted and regressed against the dependent ID measure, to determine whether the task and tool conditions obeyed Fitts’ law. Finally, we analyzed mean movement durations using a repeated-measures ANOVA with three within-subjects factors: ID (low, medium, high) × task (closed-loop, open-loop, imagery) × tool type (stick, laser). We also analyzed slopes with a second repeated-measures ANOVA in the same manner. Bonferroni-corrected post-hoc tests were used for pairwise comparisons.
Whole brain analysis
For every participant, each of the 4 fMRI runs containing closed-loop, open-loop, and imagine conditions using a stick or laser on high ID, medium ID, and low ID stimuli, were modeled separately at the first level. Orthogonal contrasts (one-tailed t-tests) were used to test for differences between each of the experimental conditions (12 s epochs) and resting baseline (12 s epochs). Orthogonal contrasts were also used to test for differences between task conditions (closed-loop > imagine, imagine > closed-loop, closed-loop > open-loop, open-loop > closed-loop, open-loop > imagine, imagine > open-loop) and for differences between tool types (stick > laser, laser > stick).
The resulting first-level contrasts of parameter estimates (COPEs) then served as inputs to higher-level analyses carried out using FLAME Stage 1 (Beckmann et al., 2003; Woolrich et al., 2004) to model and estimate random-effects components of mixed-effects variance. Z (Gaussianized T) statistic images were thresholded using a cluster-based threshold of Z > 3.1 for task and tool contrasts, as well as a whole-brain corrected cluster significance threshold of p = 0.05 (Worsley, 2001). First-level COPEs were averaged across the 4 runs for each subject separately (level 2), and then averaged across participants (level 3). For the group-level analysis, FEAT uses FMRIB’s Local Analysis of Mixed Effects (FLAME) to estimate the random-effects component of the measured inter-subject mixed-effects variance, using Markov Chain Monte Carlo sampling to get an accurate estimation of the true random-effects variance and degrees of freedom at each voxel.
Anatomical localization of brain activation was verified by manual comparison with an atlas (Duvernoy, 1991), and the multi-fiducial mapping algorithm in Caret (http://www.nitrc.org/projects/caret/) (Van Essen et al., 2001) was used to overlay group statistical maps onto a population-average, landmark- and surface-based (PALS) human brain atlas for visualization (Van Essen, 2005).
Post-hoc region-of-interest (ROI) analyses
We calculated mean percent signal change in areas of the left and right SPL that demonstrated significantly greater increases in activity for conditions involving use of the stick vs. the laser in the whole-brain analysis. We defined the boundaries of these ROIs by placing spheres with a radius of 20 mm (centered on the locations of peak activation within the left (−26, −40, 66) and right (32, −40, 66) hemispheres. We then computed mean percent signal change associated with each experimental condition relative to resting baseline across those voxels demonstrating a significant stick vs. laser effect. This was done separately within the left and right ROIs. These values were calculated separately for each participant and sub-condition using FSL’s Featquery. Repeated-measures ANOVAs were conducted to test for mean percent signal change greater than baseline as well as differences between task conditions (closed-loop vs. open-loop vs. imagine) and task difficulty (low ID vs. medium vs. high ID) within these ROIs.
Results
Behavioral results
Effect of ID
The times required for movement execution (both closed- and open-loop) and estimated for the imagery condition (i.e., movement times, MTs) increased as a linear function of task difficulty, demonstrating that both behaviors obeyed Fitts’ law. The main effect of ID on MTs was significant, F(2,38) = 11.93, p > .001. Bonferroni (Bf) corrected post-hoc tests revealed that MTs for low ID < high ID, p = .006, low ID < medium ID, p = .014, and medium ID < high ID, p = .011. Consistent with Fitts’ law, Fig. 3 shows that MTs increased as a linear function of ID. All slopes were greater than 0 (p < .05), except for the imagine stick condition (p = .176).
Fig. 3.
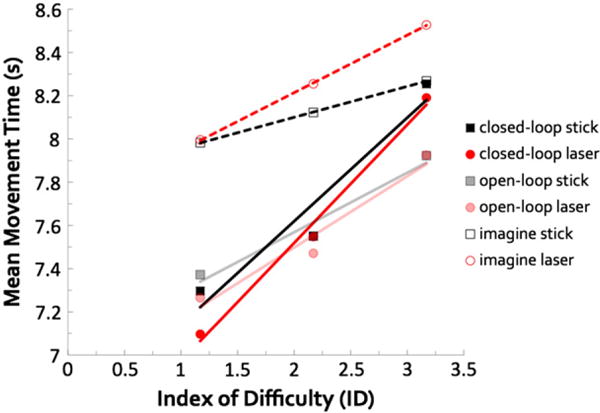
Behavioral results for the reciprocal aiming task. Mean MTs plotted against ID, calculated using Fitts’ law for all task and tool conditions. A decrease in target box size corresponds to an increase in ID, which represents task difficulty. Linear fits plotted with dark solid lines denote closed-loop movements, faded solid lines denote open-loop movements, and dashed lines denote imagined movements. The linear relationship between duration and ID (i.e., Fitts’ law) held for all conditions. MTs for low < medium < high IDs. MTs for imagine were overestimated compared to both closed-loop and open-loop. Effect of ID was more prominent for closed-loop execution. Slopes for closed-loop > both imagine and open-loop conditions. Collapsed across conditions, MTs did not differ between stick and laser, but slopes for the laser were steeper, due to the imagery conditions. All p-values < .05.
Effect of task
Speed of performance without visual feedback (open-loop) closely resembled performance during visually-guided execution (closed-loop), but differed significantly from motor imagery (imagine), indicating the importance of issuing a motor command and receiving proprioceptive feedback. Fig. 3 shows the main effect of task condition on MTs, F(2,38) = 8.81, p = .001. Post-hoc comparisons indicated that MTs for closed-loop (Bf-p = .035) and open-loop (Bf-p = .004) were shorter than for the imagine condition, but MTs for closed-loop and open-loop conditions did not differ. Slopes also differed between task conditions, F(2, 38) = 10.514, p > .001. Post-hoc comparisons showed that slopes for closed-loop > imagine (Bf-p = .002) and closed-loop > open-loop (Bf-p = .035).
We also found a task × ID interaction for MTs, F(2,38) = 3.56, p = .038. This interaction indicates that the effect of ID was most prominent for closed-loop, less so for open-loop, and smallest for imagery.
Effect of tool-type
The fact that the effects of ID on performance were similar for the stick and the laser suggests that, although differing in their end-effector properties, the dynamics of our stick and laser were well matched. Mean movement durations did not differ between the stick and the laser, F(1,19) = .01, p = .940, nor did we find an interaction between tooltype and ID F(2,38) = 2.53, p = .093. However, slopes for the stick were less steep than those for the laser, F(1,19) = 5.45, p = .031. This was entirely due to the imagine condition, as one-way repeated-measures ANOVAs of tool-type for each task condition revealed a significant, and unanticipated, difference between the stick vs. laser slopes only for the imagine condition, F(1,19) = 9.42, p = .006. This unanticipated result suggests that participants’ internal representations of movements involving the stick are less accurate than those for the laser.
fMRI results
Differences in neural activity associated with task condition
Closed-loop vs. imagine
To the extent that the SPL, IPL (Sirigu et al., 1996), and cerebellum (Grealy and Lee, 2011) are involved in feedforward control, we expected relative activity in these areas to increase when sensory feedback was removed. Consistent with dorsal– dorsal stream involvement in feedback control (Johnson and Grafton, 2003; Rizzolatti and Matelli, 2003), the SPL and interconnected regions of premotor cortex showed greater increases in activity during the closed-loop condition vs. the imagine condition. The same was observed for the cerebellum. Assuming that imagery involves feedforward control (Grush, 2004; Jeannerod, 1994), this suggests that these two regions play a greater role in feedback-based updating rather than feedforward prediction (Fig. 4, warm colors). Not surprisingly, closed-loop execution was also associated with greater increases in the left (contralateral) primary motor and sensory cortices (pre- and postcentral gyri, respectively) and in the thalamus. Earlier findings comparing executed movements vs. imagery produced similar results (Macuga and Frey, 2012). Higuchi et al. (2007) also found similar results in a tool-use study, where executed actions resulted in left sensorimotor and thalamic activations, and imagery did not. Importantly, they did not directly compare execution and imagery. Consistent with the hypothesized role of the ventral–dorsal pathway in motor planning and imagery, the IPL (bilateral angular gyrus (Ag)), exhibited greater increases in activity for the opposite contrast, imagine vs. closed-loop. Imagery also produced greater increases throughout the ventral visual stream beginning at the temporal–parietal junction and extending rostrally along the lateral temporal cortex, and in both the lateral (along the middle and inferior frontal gyri) and medial (pre-supplementary motor area (pre-SMA)) prefrontal cortex (Fig. 4, cool colors).
Fig. 4.
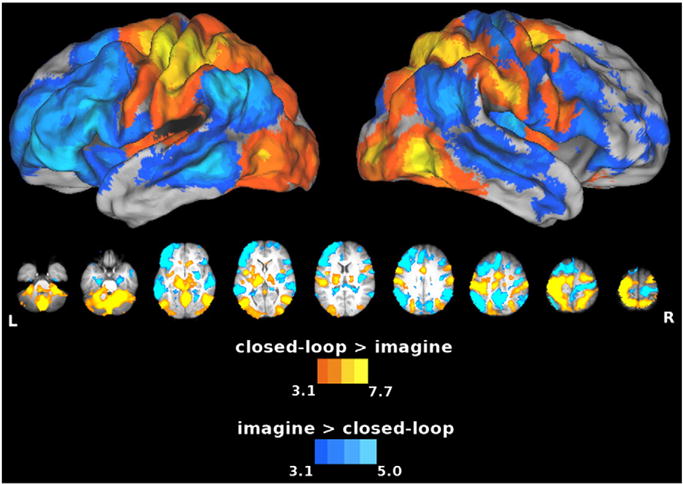
Areas showing increased activity for the closed-loop vs. imagine comparison. In this and subsequent figures, group statistical parametric maps were thresholded at Z > 3.1 (corrected clusterwise significance threshold p < 0.05). Warm colors show the closed-loop > imagine contrast, and cool colors show the imagine > closed-loop contrast. Closed-loop execution showed greater increases in activity in both the SPL and cerebellum with respect to imagery, emphasizing the importance of these areas in feedback control. On the other hand, imagery showed greater increases in activity in the IPL (Ag), left MFg, pre-SMA, MTg and dorsal striatum, areas that might be more involved in feedforward processes. Both dorsal (closed-loop)/ventral (imagery) and posterior (closed-loop)/anterior (imagery) divisions are evident here.
Closed-loop vs. open-loop
By comparing execution with visual feedback (closed-loop) to execution without visual feedback (open-loop), we were able to identify areas more involved in visually-guided vs. open loop control, where the visual feedback for performing the task was missing. Note, however, that in all three conditions, targets remained visible. Results of this comparison were strikingly similar to those for closed-loop vs. imagine, suggesting that imagery and open-loop control may indeed place demands on similar feedforward mechanisms. Relative to the open-loop condition, the SPL and cerebellum again showed greater increases in activity during the closed-loop condition, and similar effects were also detected in the left primary motor and sensory cortices (pre- and postcentral gyri, respectively), throughout the entire dorsal–dorsal stream, and in the thalamus (Fig. 5, warm colors). Conversely, as was the case for imagine vs. closed-loop (refer back to cool colors in Fig. 4), open-loop vs. closed-loop control produced increased activity in the temporal–parietal junction and throughout the entire ventral visual stream, and in the lateral and medial prefrontal cortex (including pre-SMA), as well as the dorsal striatum (Fig. 5, cool colors). This suggests that these regions may be particularly important for feedforward control and/or other compensatory cognitive processes.
Fig. 5.
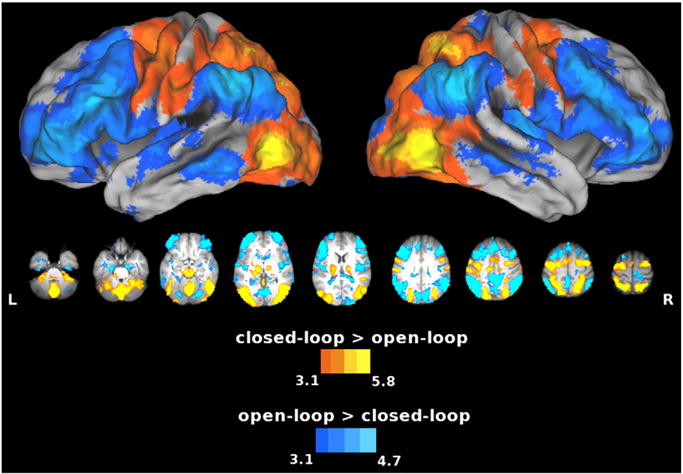
Areas showing increased activity for the closed-loop vs. open-loop comparison. Warm colors show the closed-loop > open-loop contrast, and cool colors show the open-loop > closed-loop contrast. Compared to the open-loop condition, the closed-loop condition again showed increased activation in the SPL and cerebellum. Conversely, as was the case for imagery vs. closed-loop execution (cool colors in Fig. 4), open-loop vs. closed-loop execution produced increased activity in the IPL (Ag), MFg, pre-SMA, MTg, and dorsal striatum. When visual feedback is not available, some of these areas might be involved in feedforward processes.
Open-loop vs. imagine
The absence of visual feedback in the open-loop condition was intended to increase demands on feedforward control. However, similar to the closed-loop condition, proprioceptive feedback remained available in this intermediate condition, providing some basis for feedback control. During this open-loop condition, we found greater left SPL and cerebellar activity relative to the imagine condition (Fig. 6, warm colors). Unsurprisingly, we found that the overt movements during that open-loop condition produced greater increases than imagery in the left primary motor and sensory cortices (pre- and postcentral gyri, respectively), throughout the left dorsal–dorsal stream, and in the thalamus. However, here there was increased activity in the right frontal areas, unlike results for the closed-loop > imagine contrast. The opposite contrast revealed increased activity in the left premotor and rostral middle and superior frontal gyri (MFg, SFg), rostral pre-SMA, bilateral caudal middle temporal gyri (MTg), and dorsal striatum for imagery, the condition presumably requiring greater reliance on feedforward prediction (Fig. 6, cool colors). These regions were similar to those revealed by the imagine vs. closed-loop contrast (refer back to cool colors in Fig. 4). However, the IPL difference did not appear here, nor did we detect the differences in activity along the length of the MFg.
Fig. 6.
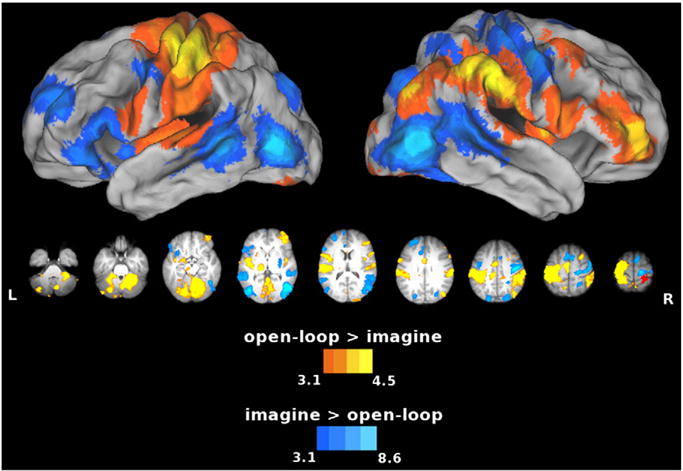
Areas showing increased activity for the open-loop vs. imagine comparison. Warm colors show the open-loop > imagine contrast, and cool colors show the imagine > open-loop contrast. The SPL and cerebellum also activated to a greater extent during open-loop execution vs. imagery, which suggests that these areas play a role in feedback control even based on proprioceptive feedback alone. The opposite contrast reveals increased activity in the left MFg, pre-SMA, MTg, and dorsal striatum (all similar for imagery vs. closed-loop execution, cool colors in Fig. 4), for imagery (the condition presumably requiring greater reliance on feedforward processes) vs. open-loop execution.
Effect of tool type on neural activity
In contrast to the hypothesis that SPOC is coding reachable space (Gallivan et al., 2009), we failed to detect differences in this region when pointing to far targets with the stick vs. laser. However, bilateral anterior SPL (putative Brodmann Area 5, Fig. 7) did show significantly greater responses during use of the stick. This result may suggest that this region codes a remapping of reachable space or distalization of the end effector when pointing with a stick but not with a laser (Arbib et al., 2009). Alternatively, it may reflect differences in demands on control of the stick vs. tool. We also saw increased activity in the left early visual cortex that is likely due to perceptual differences between the tools, specifically the shaft of the stick appearing primarily in the right visual field. By contrast, no areas showed greater responses for the laser vs. stick.
Fig. 7.
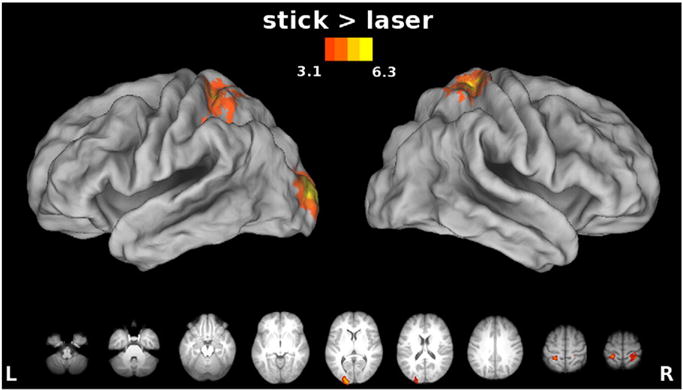
Effect of tool: stick vs. laser. The stick > laser contrast revealed greater increases in activation in bilateral SPL, putative Brodmann Area 5, suggesting a remapping of reachable space or a distalization of the end-effector when pointing with a stick but not with a laser. No significant increases in activation were found for the reverse contrast, laser > stick. We examined differences between task conditions within the SPL ROIs identified by the stick > laser contrast in Fig. 8.
SPL ROIs
Task conditions vs. baseline in the SPL ROIs
To further elucidate contributions of the SPL to tool use, an ROI analysis was undertaken in those areas that demonstrated an overall increase in activity during conditions involving use of the stick vs. the laser regardless of the condition (Fig. 7). In the left SPL, only those conditions that involved actual movements (closed-loop and open-loop) showed significantly increased activity relative to the resting baseline (p < .01 in each case) (Fig. 8A). This was not the case for left SPL responses associated with the imagine condition. Likewise, we failed to detect significant increases in right SPL activity for any of the conditions (Fig. 8B). This is reflected by the hemisphere by task interaction, F(2,38) = 22.17, p < .001, with differences between task conditions for the left, F(2,38) = 19.11, p < .001, but not for the right hemisphere. These findings suggest that the left (or contralateral) SPL may code reachable space. The fact that a stick > laser difference did not arise in the SPL for imagery suggests that some type of sensorimotor feedback is required for this distinction, or that imagery of tool use is fairly coarse and does not distinguish between tool types (Macuga et al., 2012).
Fig. 8.
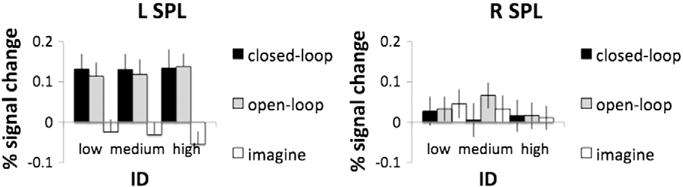
Percent signal change from the resting baseline within the SPL ROIs identified by the stick > laser contrast. Bars represent standard errors. A) For the left SPL. Only closed-loop and open-loop conditions showed activations above resting baseline. In task comparisons, closed-loop and open-loop showed greater activations than imagine, but were not significantly different from each other. B) For the right SPL. None of the conditions in the right hemisphere differed from the resting baseline or from each other. Importantly, there was no effect of ID. This, along with the behavioral results showing no interaction between tool and ID, suggests that the effect in the SPL cannot simply be explained by a difference in the difficulty of controlling the carefully matched tools.
ID levels vs. baseline in the SPL ROIs
Could the stick vs. laser difference in SPL during the conditions involving overt movements simply be explained by a difference in the difficulty of controlling the carefully matched tools? To examine this possibility, we compared percent signal change vs. baseline in the SPL ROIs across IDs (Fig. 8). Importantly, we found no effect of ID. This, along with the behavioral results showing no interaction between tool and ID, suggests that the effect in SPL cannot simply be explained by a difference in the difficulty of controlling the carefully matched tools.
Discussion
This study yielded four major findings. First, our behavioral data indicate that even when engaging in reciprocal aiming movements in the absence of visual feedback, and directed at targets in far space, Fitts’ law was upheld. This was true regardless of whether participants used a stick that extended their reach or a laser pointer that did not. However, consistent with our earlier results (Macuga et al., 2012), the complete absence of feedback and physical movement during motor imagery dampened the effect of the ID. This suggests that motor imagery may involve partially distinct cognitive mechanisms from execution for certain types of behaviors (Rodriguez et al., 2008), including those involving tools (Macuga et al., 2012). Second, the SPL and cerebellum showed greatest increases in activity during execution when vision was available for control, providing evidence for their involvement in feedback-based control. Consistent with this interpretation, we also found greater SPL and cerebellar activity for open-loop execution relative to the imagine condition. The IPL, by contrast, contributed more to the open-loop and imagine conditions in which feedback was respectively limited or absent. One possibility is that the IPL plays a greater role in feedforward control when feedback control is a less viable option. We feel that the open- vs. closed-loop comparison provides the strongest evidence, since it addresses the impact of the diminution of feedback without a substantial decrement in behavioral performance (in contrast to imagery). Third, we detected unexpected and widely distributed increases in temporal and prefrontal cortex that were significantly greater during imagery and open-loop control. Finally, we failed to find support for the hypothesis that the SPOC is involved in representing reachable space. Instead, anterior SPL responded selectively when actions directed at far targets were performed with the stick (that extends reach) vs. the laser pointer (that does not extend reach). We now consider each of these results in detail.
Fitts’ law for distal pointing with tools under different feedback conditions
As observed in previous work (Macuga et al., 2012), our behavioral findings are consistent with Fitts’ law, although the effects of ID were not as robust for the imagine condition. This latter finding suggests that the properties of tools are not fully captured in the internal representations of the limb-tool system. Specifically, MTs for imagined movements were overestimated compared to both conditions where movements were executed, which indicates that the internal representations for pointing movements with a tool are relatively coarse. On the other hand, MTs for the open-loop conditions did not differ significantly from the closed-loop MTs, suggesting that proprioceptive information can be used effectively for otherwise feedforward pointing tasks. The open-loop condition looks very similar to the closed-loop condition, though the steeper slope for closed-loop vs. both open-loop and imagery likely reflects the contribution of visual feedback to the pointing task.
Distinction between superior and inferior regions of posterior parietal cortex
Several authors have suggested that the SPL contributes more to online motor control, while the IPL plays a greater role in motor planning and cognition (Glover, 2004; Johnson and Grafton, 2003; Rizzolatti and Matelli, 2003). We did indeed find that the SPL showed much greater involvement in the closed-loop vs. either the open-loop or imagine conditions, and that the IPL exhibited more activity during the imagine vs. the closed-loop condition. Importantly, we found that when movements were executed without visual feedback, the IPL (particularly left Ag) also displayed increased activity relative to visually-guided execution. The removal of visual feedback was designed to compromise (or complicate) feedback control, while placing higher demands on feedforward control. Assuming that this manipulation was effective, then these findings suggest that the SPL and IPL are both critical for motor control but differ in their respective contributions to feedback vs. feedforward processing.
As for the hypothesis that motor imagery involves mechanisms of feedforward control (Frey, 2010; Grush, 2004; Shadmehr and Krakauer, 2008), we found considerable similarities with the open-loop condition, as well as some important distinctions. The IPL showed comparable increases in activity during the two conditions that lacked visual feedback (open-loop control and imagery). Assuming that removing visual feedback in the open loop condition was indeed effective in increasing the demands on feedforward control, then this result seems consistent with the view that imagery exploits these same mechanisms, and that the IPL is a key neural substrate. The SPL exhibited greater activity during the open-loop condition relative to imagery, which may reflect the processing of available proprioceptive feedback during movements.
These parietal areas do not act in isolation. The cerebellum appears to play a prominent role in feedback control as well, as it mirrored the results of the SPL. Others who have compared open-loop vs. imagined finger tapping found similar results (Deiber et al., 1998; Nair et al., 2003). In contrast, increasing the demands on feedforward control produced greater activity in the MTg and left MFg. Other tasks that may evoke tool-based internal representations, such as naming tools or tool use actions have been shown to selectively activate MTg (Damasio et al., 2001; Martin et al., 1996), and left MFg (Grabowski et al., 1998).
Our results also support existing evidence for a rostrocaudal functional division of the supplementary motor area (SMA), as imagery activates more anterior regions (pre-SMA) than execution (SMA) (Macuga and Frey, 2012; Picard and Strick, 1996).
Increased recruitment of prefrontal cortices during imagery and open-loop control
A striking and unexpected result was the increased engagement of lateral and medial prefrontal regions during both the imagery and open-loop conditions relative to closed loop execution. In contrast to earlier studies comparing imagery and execution of simple repetitive movements (e.g., Deiber et al., 1998; Macuga and Frey, 2012; Nair et al., 2003), the current study involved performing a demanding precision aiming task with two quite different tools across three conditions that differed substantially in their task demands. It seems feasible that these elements of the paradigm increased demands on aspects of cognitive control such as switching between conditions and action sets. Indeed, previous work implicates both the lateral (Ridderinkhof et al., 2004) and medial (Rushworth et al., 2004) prefrontal regions detected here in the cognitive control of action. For the imagery condition, the engagement of these prefrontal regions could be viewed as consistent with contributions of alternative, non-simulation strategies (Rodriguez et al., 2008; Viswanathan et al., 2012), which may also play a role in feedforward control.
Brain regions distinguishing the usage of different tools in far space
Earlier work suggests that the SPOC is involved in the representation of near space within reach of the hand (Gallivan et al., 2009). In our study, all targets were located in far space, and were reachable when using the stick, but not the laser pointer. If SPOC flexibly represents reachable space as opposed to only near space within reach of the hand, then we predicted increased activity for performance with the stick vs. laser in SPOC. We failed to detect tool-related differences within this region, but did find selective increases in activity associated with use of the stick within the rostral left SPL (putative area 5). More precisely, these effects were exclusive to the closed-loop and open-loop conditions, indicating that motor commands and/or accompanying proprioceptive feedback were key. This is consistent with involvement of the SPL in feedback-based control.
The fact that this effect occurred for execution with and without visual feedback rules out the possibility that it is due to variations in visual feedback during use of the stick vs. laser. One possible source of the tool effect in SPL is differences in the dynamics of the tools that affect their controllability. However, we verified that stick vs. laser difference in the SPL was not simply due to a difference in the difficulty of controlling the carefully matched tools by showing no effect of ID in these ROIs, which is also consistent with the behavioral results showing no interaction between tool and ID. We tentatively conclude that the rostral left SPL may instead be involved in the remapping of far into reachable (near) space (Maravita and Iriki, 2004), or in distalization of the end-effector from hand to stick (Arbib et al., 2009). Because this was an aiming task that did not involve functional interaction of the end-effector with the target objects, the spatial remapping explanation may better account for our results. While the left lateralization echoes previous results in tasks ranging from simple motor control to tool use (Johnson-Frey et al., 2005; Kroliczak and Frey, 2009), we cannot be sure whether it is a true cerebral asymmetry or simply evidence of contralateral organization, because the reciprocal aiming task was only performed with the right hand. Future studies might examine more complex, interactive tool-use tasks, such as ones that involve grasping or manipulating objects, to determine if similar mechanisms are employed in these instances.
In conclusion, our results indicate a superior-to-inferior gradient in the representation of feedback-to-feedforward control within the posterior parietal cortex. Further, they demonstrate that mechanisms involved in motor imagery overlap substantially with open-loop control, which may be attributable to the involvement of feedforward control in both conditions. Finally, we found that the anterior SPL responds selectively for actions performed with a stick, which physically extends one’s reach, as opposed to a laser pointer, which does not. We suggest that this region of the human brain supports dynamic representations of reachable, as opposed to near, space.
Acknowledgments
We thank Bill Troyer for his contributions to the LabVIEW software development, Scott Watrous for his help with fMRI scanning, and Annaleigh Boggess and Julianna Di Miceli for their assistance with running participants. A grant from NIH/NINDS (NS053962) to S.H.F. supported this work.
Footnotes
Fitts’ Law states that MT = a + b*log2(2A/W), where ‘W’ is target width, ‘A’ is amplitude, and ‘a’ and ‘b’ are empirical constants. The latter portion of this formula [log2(2A/W)] is known as the index of difficulty (ID) of the movement Importantly, this equation predicts that MT will be a linear function of the ID for various conditions within a given task However, slopes (b) may vary between tasks that involve different actions and/or effectors (Langolf et al., 1976. An investigation of Fitts’ law using a wide range of movement amplitudes. Journal of Motor Behavior 8,113–128.)
Conflict of interest
There is no conflict of interest.
References
- Andersson JLR, Jenkinson M, Smith SM. Non-linear Optimisation 2007 [Google Scholar]
- Arbib MA, Bonaiuto JB, Jacobs S, Frey SH. Tool use and the distalization of the end-effector. Psychol Res. 2009;73:441–462. doi: 10.1007/s00426-009-0242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann C, Smith S. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging. 2004;23:137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in fMRI. Neuroimage. 2003;20:1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Berti A, Frassinetti F. When far becomes near: remapping of space by tool use. J Cogn Neurosci. 2000;12:415–420. doi: 10.1162/089892900562237. [DOI] [PubMed] [Google Scholar]
- Blakemore S, Sirigu A. Action prediction in the cerebellum and in the parietal lobe. Exp Brain Res. 2003;153:239–245. doi: 10.1007/s00221-003-1597-z. [DOI] [PubMed] [Google Scholar]
- Damasio H, Grabowski TJ, Tranel D, Ponto LL, Hichwa RD, Damasio AR. Neural correlates of naming actions and of naming spatial relations. Neuroimage. 2001;13:1053–1064. doi: 10.1006/nimg.2001.0775. [DOI] [PubMed] [Google Scholar]
- Deiber MP, Ibanez V, Honda M, Sadato N, Raman R, Hallett M. Cerebral processes related to visuomotor imagery and generation of simple finger movements studied with positron emission tomography. Neuroimage. 1998;7:73–85. doi: 10.1006/nimg.1997.0314. [DOI] [PubMed] [Google Scholar]
- Desmurget M, Grafton S. Forward modeling allows feedback control for fast reaching movements. Trends Cogn Sci. 2000;4:423–431. doi: 10.1016/s1364-6613(00)01537-0. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM. The Human Brain: Surface, Blood Supply, and Three-dimensional Sectional Anatomy. 2nd. Springer Wien; New York: 1991. [Google Scholar]
- Fitts PM. The information capacity of the human motor system in controlling the amplitude of movement. J Exp Psychol. 1954;47:381–391. [PubMed] [Google Scholar]
- Frey SH. Forecasting the long-range consequences of manual and tool use actions: neurophysiological, behavioral and computational considerations. In: Danion FMLE, editor. Motor Control: Theories, Experiments and Applications. New York, Oxford: 2010. pp. 295–313. [Google Scholar]
- Frey SH, Fogassi L, Grafton S, Picard N, Rothwell JC, Schweighofer N, Corbetta M, Fitzpatrick SM. Neurological principles and rehabilitation of action disorders: computation, anatomy, and physiology (CAP) model. Neurorehabil Neural Repair. 2011;25:6S–20S. doi: 10.1177/1545968311410940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallivan JP, Cavina-Pratesi C, Culham JC. Is that within reach? fMRI reveals that the human superior parieto-occipital cortex encodes objects reachable by the hand. J Neurosci. 2009;29:4381–4391. doi: 10.1523/JNEUROSCI.0377-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover S. Separate visual representations in the planning and control of action. Behav Brain Sci. 2004;27:3–24. doi: 10.1017/s0140525x04000020. (discussion 24–78) [DOI] [PubMed] [Google Scholar]
- Gonzalez B, Rodriguez M, Ramirez C, Sabate M. Disturbance of motor imagery after cerebellar stroke. Behav Neurosci. 2005;119:622–626. doi: 10.1037/0735-7044.119.2.622. [DOI] [PubMed] [Google Scholar]
- Grabowski TJ, Damasio H, Damasio AR. Premotor and prefrontal correlates of category-related lexical retrieval. Neuroimage. 1998;7:232–243. doi: 10.1006/nimg.1998.0324. [DOI] [PubMed] [Google Scholar]
- Grealy MA, Lee DN. An automatic-voluntary dissociation and mental imagery disturbance following a cerebellar lesion. Neuropsychologia. 2011;49:271–275. doi: 10.1016/j.neuropsychologia.2010.09.031. [DOI] [PubMed] [Google Scholar]
- Grush R. The emulation theory of representation: motor control, imagery, and perception. Behav Brain Sci. 2004;27:377–396. doi: 10.1017/s0140525x04000093. (discussion 396–442) [DOI] [PubMed] [Google Scholar]
- Higuchi S, Imamizu H, Kawato M. Cerebellar activity evoked by common tool-use execution and imagery tasks: an fMRI study. Cortex. 2007;43:350–358. doi: 10.1016/s0010-9452(08)70460-x. [DOI] [PubMed] [Google Scholar]
- Imamizu H, Miyauchi S, Tamada T, Sasaki Y, Takino R, Putz B, Yoshioka T, Kawato M. Human cerebellar activity reflecting an acquired internal model of a new tool. Nature. 2000;403:192–195. doi: 10.1038/35003194. [DOI] [PubMed] [Google Scholar]
- Iriki A, Tanaka M, Iwamura Y. Coding of modified body schema during tool use by macaque postcentral neurones. Neuroreport. 1996;7:2325–2330. doi: 10.1097/00001756-199610020-00010. [DOI] [PubMed] [Google Scholar]
- Ito M. The Cerebellum and Neural Control. Raven Press; New York: 1984. [Google Scholar]
- Jeannerod M. The representing brain: neural correlates of motor intention and imagery. Brain Behav Sci. 1994;17:187–245. [Google Scholar]
- Jenkinson M. Fast, automated, N-dimensional phase-unwrapping algorithm. Magn Reson Med. 2003;49:193–197. doi: 10.1002/mrm.10354. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Johnson SH, Grafton S. From ‘acting on’ to ‘acting with’: the functional anatomy of object-oriented action schemata. Prog Brain Res. 2003;142:127–139. doi: 10.1016/S0079-6123(03)42010-4. [DOI] [PubMed] [Google Scholar]
- Johnson-Frey SH, Newman-Norlund R, Grafton S. A distributed left hemisphere network active during planning of everyday tool use skills. Cereb cortex. 2005;15:681–695. doi: 10.1093/cercor/bhh169. (New York, NY: 1991) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawato M. Internal models for motor control and trajectory planning. Curr Opin Neurobiol. 1999;9:718–727. doi: 10.1016/s0959-4388(99)00028-8. [DOI] [PubMed] [Google Scholar]
- Kording KP, Wolpert DM. Bayesian decision theory in sensorimotor control. Trends Cogn Sci. 2006;10:319–326. doi: 10.1016/j.tics.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Kroliczak G, Frey S. A common network in the left cerebral hemisphere represents planning of tool use pantomimes and familiar intransitive gestures at the hand-independent level. Cereb Cortex. 2009;15 doi: 10.1093/cercor/bhn261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langolf GD, Chaffin DB, Foulke JA. An investigation of Fitts’ law using a wide range of movement amplitudes. J Mot Behav. 1976;8:113–128. doi: 10.1080/00222895.1976.10735061. [DOI] [PubMed] [Google Scholar]
- Longo MR, Lourenco SF. On the nature of near space: effects of tool use and the transition to far space. Neuropsychologia. 2006;44:977–981. doi: 10.1016/j.neuropsychologia.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Macuga KL, Frey SH. Neural representations involved in observed, imagined, and imitated actions are dissociable and hierarchically organized. Neuroimage. 2012;59:2798–2807. doi: 10.1016/j.neuroimage.2011.09.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macuga K, Papailiou A, Frey S. Motor imagery of tool use: relationship to actual use and adherence to Fitts’ law across tasks. Exp Brain Res. 2012;218:11. doi: 10.1007/s00221-012-3004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maravita A, Iriki A. Tools for the body (schema) Trends Cogn Sci. 2004;8:79–86. doi: 10.1016/j.tics.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Martin A, Wiggs CL, Ungerleider LG, Haxby JV. Neural correlates of category-specific knowledge. Nature. 1996;379:649–652. doi: 10.1038/379649a0. [DOI] [PubMed] [Google Scholar]
- Nair DG, Purcott KL, Fuchs A, Steinberg F, Kelso JA. Cortical and cerebellar activity of the human brain during imagined and executed unimanual and bimanual action sequences: a functional MRI study. Brain Res Cogn Brain Res. 2003;15:250–260. doi: 10.1016/s0926-6410(02)00197-0. [DOI] [PubMed] [Google Scholar]
- Pegna AJ, Petit L, Caldara-Schnetzer AS, Khateb A, Annoni JM, Sztajzel R, Landis T. So near yet so far: neglect in far or near space depends on tool use. Ann Neurol. 2001;50:820–822. doi: 10.1002/ana.10058. [DOI] [PubMed] [Google Scholar]
- Picard N, Strick PL. Motor areas of the medial wall: a review of their location and functional activation. Cereb Cortex. 1996;6:342–353. doi: 10.1093/cercor/6.3.342. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, van den Wildenberg WP, Segalowitz SJ, Carter CS. Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cogn. 2004;56:129–140. doi: 10.1016/j.bandc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Matelli M. Two different streams form the dorsal visual system: anatomy and functions. Exp Brain Res. 2003;153:146–157. doi: 10.1007/s00221-003-1588-0. [DOI] [PubMed] [Google Scholar]
- Rodriguez M, Llanos C, Gonzalez S, Sabate M. How similar are motor imagery and movement? Behav Neurosci. 2008;122:910–916. doi: 10.1037/0735-7044.122.4.910. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Walton ME, Kennerley SW, Bannerman DM. Action sets and decisions in the medial frontal cortex. Trends Cogn Sci. 2004;8:410–417. doi: 10.1016/j.tics.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Seidler RD, Noll DC, Thiers G. Feedforward and feedback processes in motor control. Neuroimage. 2004;22:1775–1783. doi: 10.1016/j.neuroimage.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Krakauer J. A computational neuroanatomy for motor control. Exp Brain Res. 2008;185:359–381. doi: 10.1007/s00221-008-1280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirigu A, Duhamel JR, Cohen L, Pillon B, Dubois B, Agid Y. The mental representation of hand movements after parietal cortex damage. Science. 1996;273:1564–1568. doi: 10.1126/science.273.5281.1564. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl. 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Todorov E. Optimality principles in sensorimotor control. Nat Neurosci. 2004;7:907–915. doi: 10.1038/nn1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC. A population-average, landmark- and surface-based (PALS) atlas of human cerebral cortex. Neuroimage. 2005;28:635–662. doi: 10.1016/j.neuroimage.2005.06.058. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Drury HA, Dickson J, Harwell J, Hanlon D, Anderson CH. An integrated software suite for surface-based analyses of cerebral cortex. J Am Med Inform Assoc. 2001;8:443–459. doi: 10.1136/jamia.2001.0080443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan S, Fritz C, Grafton ST. Telling the right hand from the left hand: multisensory integration, not motor imagery, solves the problem. 2012;23:598–607. doi: 10.1177/0956797611429802. [DOI] [PubMed] [Google Scholar]
- Winstein CJ, Grafton ST, Pohl PS. Motor task difficulty and brain activity: investigation of goal-directed reciprocal aiming using positron emission tomography. J Neurophysiol. 1997;77:1581–1594. doi: 10.1152/jn.1997.77.3.1581. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Flanagan JR. Motor prediction. Curr Biol. 2001;11:R729–R732. doi: 10.1016/s0960-9822(01)00432-8. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Goodbody SJ, Husain M. Maintaining internal representations: the role of the human superior parietal lobe. Nat Neurosci. 1998a;1:529–533. doi: 10.1038/2245. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Miall RC, Kawato M. Internal models in the cerebellum. Trends Cogn Sci. 1998b;2:338–347. doi: 10.1016/s1364-6613(98)01221-2. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage. 2004;21:1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Worsley KJ. Statistical analysis of activation images. In: Jezzard P, Matthews PM, Smith SM, editors. Functional MRI: An Introduction to Methods. Oxford University Press; USA: 2001. pp. 251–270. [Google Scholar]


