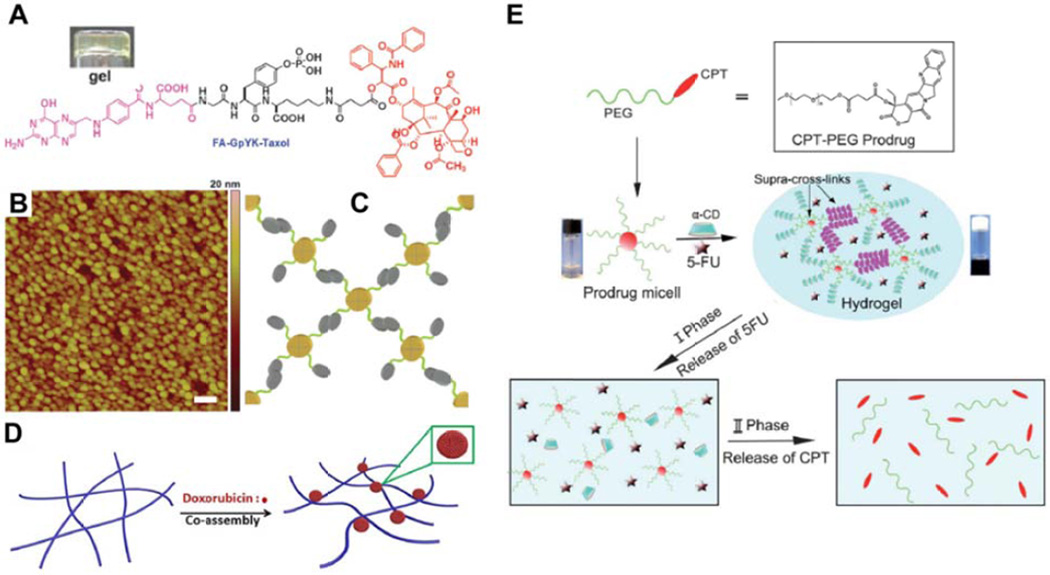
Figure 9.
Alternate approaches to the creation of molecular hydrogelators. (A–C) Yang’s folic acid (FA)-taxol based hydrogelator (A) that forms nanospheres rather than nanofilaments, as indicated by atomic force microscopy (AFM) (B), through Hoogsteen hydrogen bonding between FA molecules (C). Futher interactions between these nanospheres induces the formation of a weak gel. Adapted with permission from ref [170], copyright 2011 Royal Society of Chemistry. (D) Schematic illustration of Xue’s platform, in which a non-gelating peptide is induced to form a hydrogel through the formation of doxorubicin nanospheres that act as cross-linkers between filaments. Reprinted with permission from ref [171], copyright 2015 Nature Publishing Group. (E) Schematic illustration of the highly supramolecular approach adopted by Shi, in which CPT-PEG micelles form polypseudorotaxane structures via threading of α-cyclodextrin onto one or more PEG chains, thereby cross-linking and triggering gel formation. The drug 5-fluorouracil (5-FU) could also be encapsulated within the gel, with the slow dethreading of the psuedorotaxane breaking down the gel and releasing 5-FU and PEG-CPT micelles, that then underwent hydrolysis to release CPT. Reprinted from ref [173], copyright 2013 Royal Society of Chemistry.