Figure 4. Gαi2 directly interacts with β-pix in invadopodia.
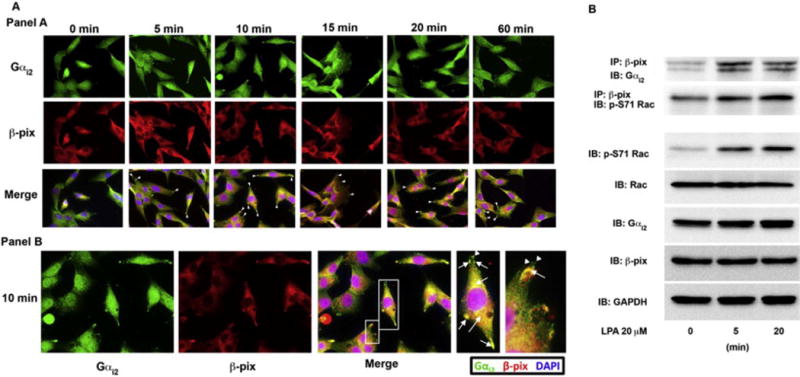
(A) Panel A: Gαi2 co-localizes with β-pix in invadopodia. To determine if Gαi2 and β-pix interact in invadopodia we treated HeyA8 with LPA for the indicated time points and stained the cells with anti-β-pix and anti-Gαi2 antibodies. Gαi2 and β-pix were found to co-localize in the leading edge during all time points tested. Panel B: The 10-minute image from panel A is shown. Zoomed in image of merged image for the 10-minute time point (white boxes) showing co-localization of Gαi2 and β-pix in invadopodia (arrows) both in the leading edge and in the perinuclear region. β-pix was also found to also localize to focal adhesions (arrowhead) without Gαi2. (B) Gαi2 and β-pix were shown to directly interact after LPA stimulation of 5 minutes and 20 minutes. β-pix and phosphorylated Rac were also shown to interact at the same time points. Phosphorylated Rac correlated with Rac activation seen by stimulation of these cells with LPA in Figure 3C and in the subsequent imaging studies. HeyA8 cells were plated and serum-starved for 18 hours. The cells were then left untreated or stimulated with 20 μM of LPA for 10 minutes, lysed and 500 μg of protein from the lysate was immunoprecipitated for β-pix for 24 hours. After immunoprecipitation, the lysate was separated by 10% SDS-PAGE and immunoblotted for Gαi2 and activated Rac, which was phosphorylated at Ser-71 using specific antibodies (p-S71 Rac). Total cell lysates were analyzed for phosphorylated Rac (p-S71 Rac), Rac1, β-pix, and Gαi2 along with GAPDH as a loading control.
