Abstract
Background
Exposure to antenatal stressors affects autonomic regulation in fetuses. Whether the presence of congenital heart disease (CHD) alters the developmental trajectory of autonomic regulation is not known.
Aims/Study Design
This prospective observational cohort study aimed to further characterize autonomic regulation in fetuses with CHD; specifically hypoplastic left heart syndrome (HLHS), transposition of the great arteries (TGA), and tetralogy of Fallot (TOF).
Subjects
From 11/2010 – 11/2012, 92 fetuses were enrolled: 41 controls and 51 with CHD consisting of 19 with HLHS, 12 with TGA, and 20 with TOF. Maternal abdominal fetal electrocardiogram (ECG) recordings were obtained at 3 gestational ages: 19-27 weeks (F1), 28-33 weeks (F2), and 34-38 weeks (F3).
Outcome measures
Fetal ECG was analyzed for mean heart rate along with 3 measures of autonomic variability of the fetal heart rate: interquartile range, standard deviation, and root mean square of the standard deviation of the heart rate (RMSSD), a measure of parasympathetic activity.
Results
During F1 and F2 periods, HLHS fetuses demonstrated significantly lower mean HR than controls (p<0.05). Heart rate variability at F3, as measured by standard deviation, interquartile range, and RMSSD was lower in HLHS than controls (p<0.05). Other CHD subgroups showed a similar, though non-significant trend towards lower variability.
Conclusions
Autonomic regulation in CHD fetuses differs from controls with HLHS fetuses most markedly affected.
Introduction
The autonomic nervous system is key to mounting responses to physiologic stress. Development of autonomic function follows a predictable pattern as gestation progresses with alterations in this pattern associated with impaired neurological outcomes (1). Changes in autonomic regulation can be measured by markers, such as fluctuations in fetal heart rate (1), and these changes have been associated with neurodevelopmental outcomes in infants (2). Using these markers, DiPietro et al. recently reported maternal pregnancy-specific psychological stress was associated with changes in fetal neurological maturation (3). We have noted alterations in perinatal autonomic functioning in response to fetal exposure to alcohol, smoking, and maternal depression (4, 5). The proposed mechanism for these autonomic abnormalities includes brainstem changes in cardiac and respiratory regulation, but details of these alterations have not yet been elucidated (5). Changes in fetal blood flow secondary to structural heart disease can be considered a stressor that may impact ANS development.
While studies on fetal autonomic regulation have recently emerged, there is scarce literature on fetal autonomic regulation among CHD survivors. Congenital heart disease (CHD) is the most common group of congenital abnormalities affecting roughly 1% of live-born infants (6). Infants with CHD have been found to have abnormal heart rate variability and decreased heart rate response likely resulting from a combination of pre-operative, fetal, and surgical factors (7). This study aims to further characterize autonomic regulation in fetuses with CHD; specifically hypoplastic left heart syndrome (HLHS), transposition of the great arteries (TGA), and tetralogy of Fallot (TOF). We predict CHD fetuses will be more likely to demonstrate abnormal autonomic regulation compared with controls as measured by autonomic markers on fetal electrocardiogram.
Methods
Subjects
Control fetuses with normal cardiac structure, as well as fetuses with HLHS, TGA, or TOF, as diagnosed on fetal echocardiogram, were the subjects of this prospective observational cohort study. HLHS is the most severe defect of the three, characterized by underdevelopment of the left side of the heart with resultant single ventricle physiology and diminished delivery of blood to the body. TOF consists of an anatomic defect on the right side of the heart that results in obstruction of blood flow to the lungs and therefore diminished oxygenation. In TGA, the two great arteries that leave the heart, one to the body and one to the lungs, are ‘switched’ resulting in the left heart receiving oxygenated blood from the lungs and cycling it back to the lungs while the right heart receives deoxygenated blood from the body and pumps it back to the vital organs. All three cardiac lesions require surgery within the first year of life for survival.
Participants were less than 24 weeks gestational age (GA) at enrollment. Exclusion criteria included multiple gestation, evidence of chromosomal abnormalities, structural brain malformations, placental insufficiency, intrauterine growth retardation or sustained cardiac arrhythmia. The Columbia University Medical Center Institutional Review Board approved this study. Written informed consent was obtained from pregnant mothers.
Measuring autonomic activity
Fetal electrocardiogram (fECG) was used to record fetal heart rate. Using electrodes placed on the maternal abdomen, the Monica AN24 monitor provided non-invasive and more accurate measures of fetal heart rate (FHR) and heart rate variability (HRV), compared with traditional Doppler FHR monitors due to the increased sampling rate from 4Hz in Doppler to 300-900 Hz in the Monica. In addition, the precise measurement of R-R intervals by Monica DK allowed for the calculation of beat to beat variability, i.e. the root mean square of the standard deviation of the heart rate, which was previously only obtainable with fetal scalp electrodes during labor.
Fetal ECG measurements were obtained from enrolled participants at 3 gestational ages: 19-27 (F1), 28-33 (F2), and 34-38 (F3) weeks. Maternal recordings lasted 50 minutes to ensure both active and quiet fetal states were obtained. In addition to R-R interval, 3 measures of fetal autonomic variability were calculated from fECG data: 1) interquartile range of the FHR (IQR) 2) standard deviation of the FHR (SD) and 3) root mean square of the standard deviation of the heart rate (RMSSD), a time domain measure of beat-to-beat heart rate variability.
Fetal state coding
Recent studies suggest fetal state as determined by active vs. quiet sleep can significantly impact HRV, particularly later in pregnancy when fetal activity is greatest (8). To differentiate between active states (REM sleep and active waking) and quiet fetal states (quiet sleep and quiet waking) in F3 (34-38 weeks GA), we measured fetal movement using a Toitu maternal abdominal Doppler ultrasound monitor. Recordings were analyzed in 5 minute intervals.
Quality assurance and statistical analysis
Prior to analysis, we assessed data quality. Fetal ECG studies met criteria as outliers if mean HR in any 5 minute interval was greater than 170 bpm or less than 100 bpm. Data quality was defined by the percentage of R-R intervals marked, as well as the percentage of epochs with FHR data on the fECG. Studies with less than 10% of marked R-R intervals and less than 15% of epochs with FHR data were considered poor quality. Data are reported as medians with interquartile range. Correlations between HRV measures and gestational age were assessed using Pearson's correlation coefficient. Statistical analysis of FHR and the aforementioned measures of HRV were compared between control and CHD fetuses using nonparametric Wilcoxon and Kruskal-Wallis tests. Alpha values were set at 0.05.
Results
Over 24 months, 92 subjects were enrolled: 41 controls and 51 CHD fetuses including 19 with HLHS, 12 with TGA, and 20 with TOF (Table I). Following data quality analysis, 117 of the 196(60%) fECG recordings were found to have high data quality, with no difference in data quality between control and CHD fetuses (p=0.799).
Table 1.
Number of complete studies with good data quality by diagnosis and gestational age group. HLHS = hypoplastic left heart syndrome, TGA = transposition of the great arteries, TOF = tetralogy of fallot.
| Gestational Age | ||||
|---|---|---|---|---|
| 19 0/7- 27 6/7 wks (F1) | 28 0/7-33 6/7 wks (F2) | 34 0/7-38 6/7wks (F3 state coded) | ||
| Diagnosis | Control | 23 | 13 | 13 |
| HLHS | 9 | 5 | 6 | |
| TGA | 8 | 5 | 9 | |
| TOF | 5 | 9 | 5 | |
| Total | 43 | 32 | 33 | |
Analysis by diagnostic subgroup across gestational age
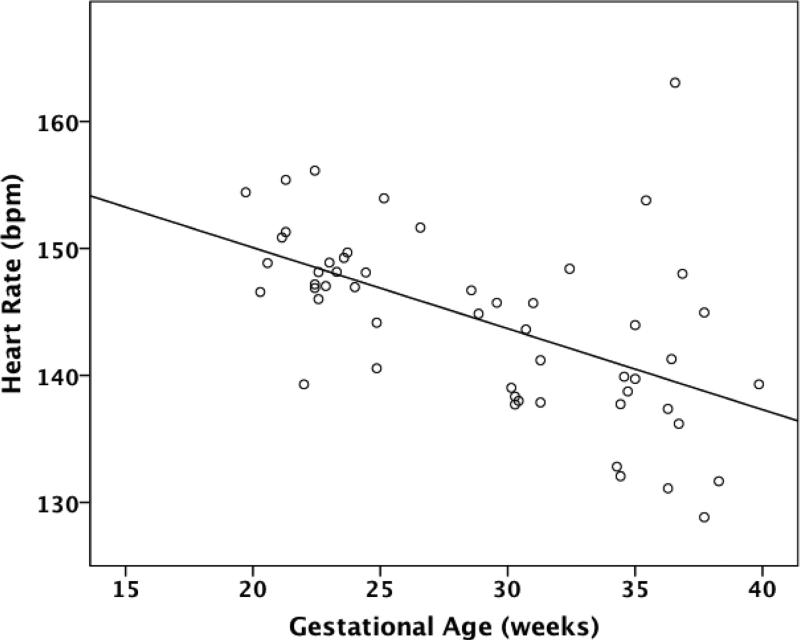
Across GA, control fetuses demonstrated a significant negative correlation with heart rate (r=-0.56, p<0.001, Figure 1). Controls also demonstrated a significant positive correlation with SD and IQR (p<0.001) as has been previously reported (5). Similar to control fetuses, CHD fetuses as a group demonstrated significant positive correlations with SD (r=0.414) and IQR (r=0.424) and gestational age (p<0.001), signifying heart rate variability increases with advancing gestation.
Figure 1.
Correlation between heart rate and gestational age in control fetuses. bpm=beats per minute
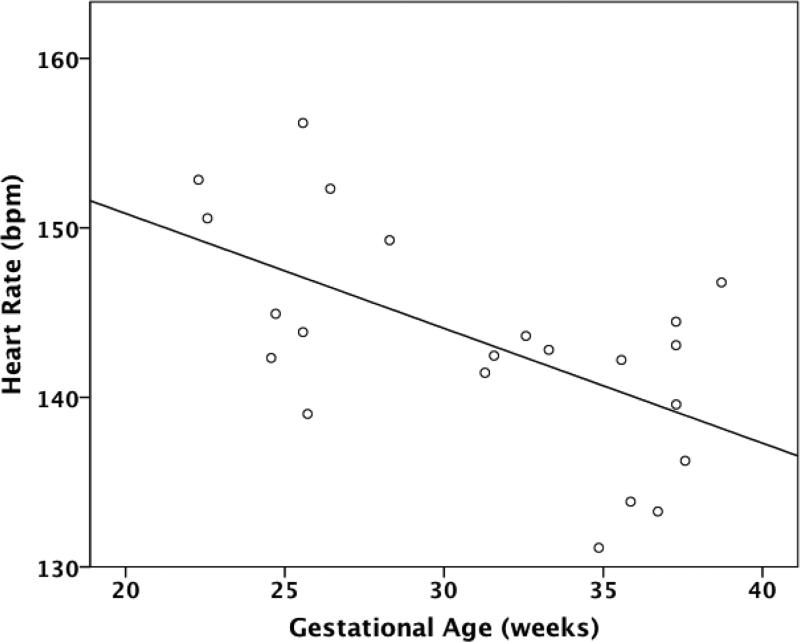
In subgroup analysis, TGA fetuses were the only CHD type to follow the same pattern seen in controls of decreasing FHR and increasing SD and IQR across GA (Figure 4). TOF fetuses demonstrated significantly increasing IQR with GA (r=0.459, p=0.042). Notably, HLHS fetuses did not demonstrate any significant correlations between any measures of FHR or HRV and GA.
Figure 4.
Standard Deviation of the fetal heart rate in control vs. hypoplastic left heart syndrome (HLHS) fetuses in the active fetal state during the gestational group from 34 to 38 weeks gestation.
Analysis by gestational age group
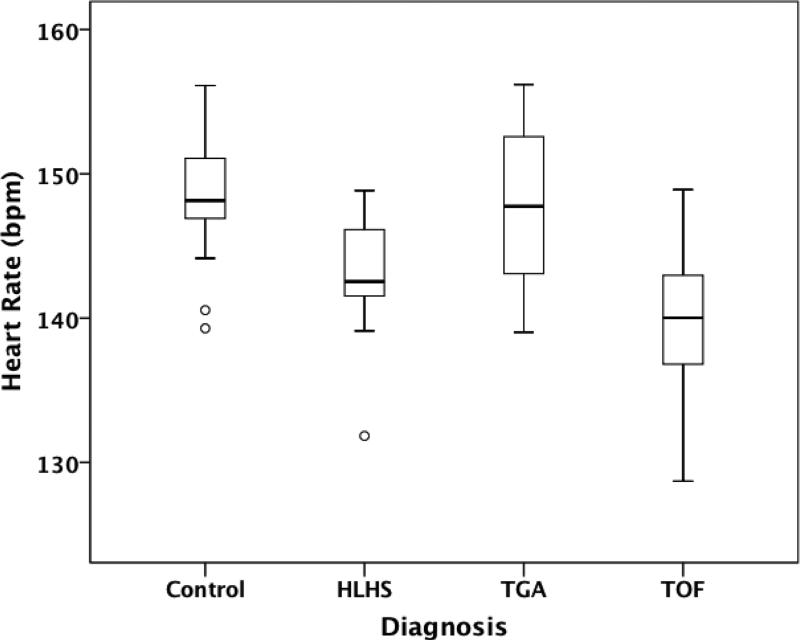
In F1 (19-27 weeks GA), CHD fetuses demonstrated significantly lower HR than controls [143(IQR 137-148) vs. 148(IQR 146-150) bpm; p=0.007). In subgroup analysis, the lowest HR was found in TOF [140 (IQR 133-147) bpm; p=0.013] and HLHS subjects [143 (IQR 140-146) bpm; p=0.002] (Figure 1). Heart rate variability (SD, RMSSD, IQR) did not differ significantly between control and CHD groups at F1.
At F2 (28-33 weeks GA), HR in HLHS fetuses remained lower than controls [136 (IQR 131-141) vs. 141(IQR 137-144) bpm; p=0.016]. Subgroup analysis did not demonstrate any significant differences in measures of HRV at this age.
Fetal state coding
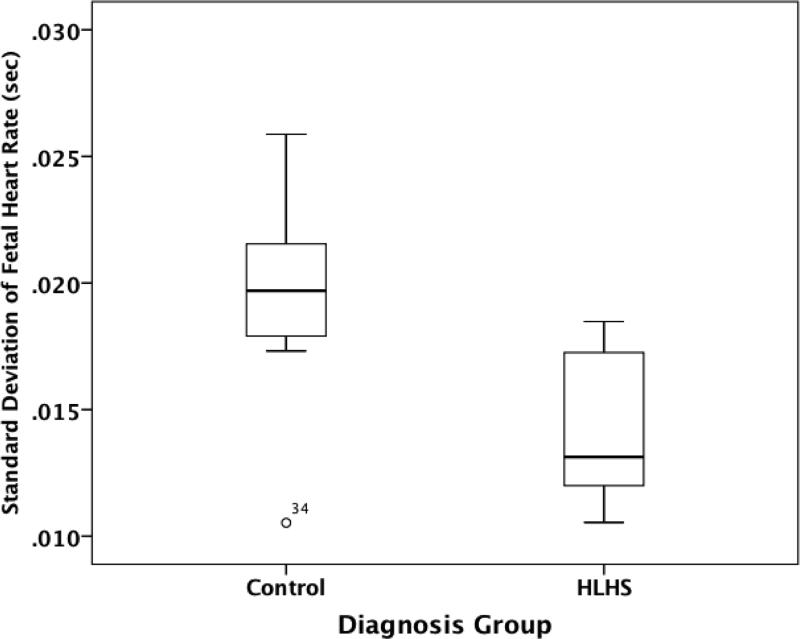
At F3 (34-38 weeks GA), significant differences in HRV were found between HLHS and control fetuses during the active fetal state. In the active state, HLHS fetuses were found to have significantly lower SD than controls [0.013 (IQR 0.010-0.016) vs. 0.020 (IQR 0.018-0.022) seconds; p=0.013], (Figure 2). Similarly, IQR was significantly lower in HLHS fetuses than controls [0.016 (IQR 0.012-0.020)] vs. 0.026 (IQR 0.022-0.030) seconds; p=0.032]. RMSSD was significantly lower in HLHS compared with controls [0.012 (IQR 0.010-0.014) vs. 0.017 (IQR 0.014-0.020) seconds; p=0.041]. No significant differences were found between controls and other CHD subgroups in the active or quiet fetal state.
Figure 2.
Correlation between heart rate and gestational age in fetuses with transposition of the great arteries (TGA). bpm=beats per minute
Discussion
This study demonstrates that autonomic nervous system regulation and development in CHD fetuses differs from controls with differences detectable as early as 19 weeks gestational age. Overall, CHD fetuses demonstrated higher fetal heart rates and lower levels of heart rate variability compared with controls. In adults, studies have shown decreased heart rate variability is associated with worse survival among patients with cardiovascular diseases (9). Fluctuations in fetal autonomic activity have been established as reliable markers of central nervous system development and associated with alterations in neurocognitive outcomes (10, 1, 2, 3) suggesting changes in fetal heart rate variability could identify CHD fetuses at risk for poor neurodevelopment. Fetal ECG monitoring is simple, non-invasive, and low cost. Our preliminary findings indicate fetal heart rate monitoring holds promise as a valuable and accessible tool of early-risk assessment in the high-risk CHD population.
The difference in autonomic activity found in CHD fetuses was most apparent among subjects with HLHS, the group with the most severe type of congenital defect both in terms of overall mortality as well as morbidity such as impaired neurobehavioral outcomes (11). While mean HR was quite low in HLHS fetuses during the F1 period of 19 to 27 weeks, the decreased variability in this group was most notable during F3 (34 to 38 weeks). TGA and TOF fetuses also appeared to have decreased autonomic variability compared with controls with the most marked differences in F2 and F3 (28 to 38 weeks gestation), though these differences were statistically non-significant, possibly due to low sample size.
Control and TGA fetuses displayed a similar trajectory for heart rate variability across gestational age, suggesting the overall blueprint for autonomic development may not vary significantly between these groups. The notable exception again was the group with HLHS: subjects with HLHS did not demonstrate any significant change in heart rate variability across gestational age. These results suggest the overall balance between sympathetic and parasympathetic activity in HLHS fetuses is different than controls.
These findings in concert suggest autonomic development in CHD fetuses may begin to deviate from a normal trajectory early in fetal development. These changes may translate into variable autonomic regulation in the term newborn, as has been documented in previous studies (7). As suggested by prior investigations of fetal stressors and autonomic regulation, the etiology of the decreased variability in CHD fetuses is likely multifactorial. The structural changes in circulation that accompany CHD, including alterations in cerebral blood flow, may impact brainstem regulation of autonomic activity (6, 13). Underlying genetic factors may also contribute. The exact mechanisms underlying differences in ANS function remain speculative.
Limitations and Future Directions
This study has limitations that provide opportunity for further exploration. Our sample size was modest. In addition, the low quality data obtained from some subjects, primarily a function of relatively brief study durations, further limited the number of data points available for analysis. Throughout the study, we implemented changes to improve fECG quality, such as lengthening fetal heart rate recording time, with significantly improved success. Fetal breathing movements have been found to increase HRV, especially in the latter half of the third trimester, and may have affected the results of this study (12). Coupling the fECG with the fetal movement signal could control for the variability attributable to the movement signal in future studies.
Conclusion
Autonomic regulation in CHD fetuses, including HLHS, TGA, and TOF, differs from controls. HLHS fetuses, which have the most severe form of CHD, were found to have the lowest heart rate variability, particularly in late gestation. Investigation is ongoing to determine if these differences in autonomic regulation among CHD fetuses translate into differences in neurodevelopmental outcome.
Highlights.
Hypoplastic left heart fetuses demonstrate lower mean heart rate than controls
Heart rate variability in hypoplastic left heart fetuses is lower than controls
Other congenital heart disease subgroups trend towards lower heart rate variability
Figure 3.
Mean heart rate in controls vs. congenital heart disease subgroups during the gestational group from 19 to 27 weeks. HLHS= Hypoplastic Left Heart Syndrome, TGA= Transposition of the Great Arteries, TOF = Tetralogy of Fallot, bpm= beats per minute
Acknowledgments
IA Williams received support from Grant #1K23HD061601 from the NICHD/NIH as well as from Grant # KL2 RR024157 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. We thank the Fifer Lab and Irving Center for their guidance and technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: The authors report no conflict of interest.
References
- 1.Hoyer D, Heinicke E, Jaekel S, Tetschke F, Paolo DD, Haueisen J, Schleubner E, Schneider U. Indices of fetal development derived from heart rate patterns. Early Hum Dev. 2009 Jan;85:379–386. doi: 10.1016/j.earlhumdev.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 2.DiPietro JA, Bornstein MH, Hahn CS, Costigan K, Achy-Brou A. Fetal heart rate and variability: stability and prediction to developmental outcomes in early childhood. Child Dev. 2007 Nov-Dec;78(6):1788–98. doi: 10.1111/j.1467-8624.2007.01099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DiPietro JA, Kivlighan KT, Costigan KA, Rubin SE, Shiffler DE, Henderson JL, Pillion JP. Prenatal antecedents of newborn neurological maturation. Child Dev. 2010 Jan-Feb;81(1):115–130. doi: 10.1111/j.1467-8624.2009.01384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fifer WP, Fingers ST, Youngman M, Gomez-Gribben E, Myers MM. Effects of alcohol and smoking during pregnancy on infant autonomic control. Dev Psychobiol. 2009;51(3):234–242. doi: 10.1002/dev.20366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monk C, Sloan RP, Myers MM, Ellman L, Werner E, Jeon J, Tager F, Fifer WP. Fetal heart rate reactivity differs by women's psychiatric status: an early marker for developmental risk? J Am Acad Child Adolesc Psychiatry. 2004;43(3):283–90. doi: 10.1097/00004583-200403000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Fyler DC. Prevalence. In: Fyler DC, editor. Nadas’ Pediatr Cardiol. Hanley & Belfus, Inc.; Philadelphia, PA: 1992. p. 273. [Google Scholar]
- 7.Kaltman JR, Hanna BD, Gallagher PR, Gaynor JW, Godinez RI, Tanel RE, Shah MJ, Vetter VL, Rhodes LA. Heart rate variability following neonatal heart surgery for complex congenital heart disease. Pacing and Clin Electrophysiol. 2006;29:471–478. doi: 10.1111/j.1540-8159.2006.00378.x. [DOI] [PubMed] [Google Scholar]
- 8.Schneider U, Frank B, Fiedler A, Kaehler C, Hoyer D, Liehr M, Haueisen J, Schleussner E. Human fetal heart rate variability-characteristics of autonomic regulation in the third trimester of gestation. J Perinat. Med. 2008;36:433–441. doi: 10.1515/JPM.2008.059. [DOI] [PubMed] [Google Scholar]
- 9.Hoyer D, Maestri R, La Rovere MT, Pinna GD. Autonomic response to cardiac dysfunction in chronic heart failure: a risk predictor based on autonomic information flow. Pacing and Clin Electrophysiol. 2008;31(2):214–220. doi: 10.1111/j.1540-8159.2007.00971.x. [DOI] [PubMed] [Google Scholar]
- 10.Porges SW. The polyvagal perspective. Biol Psychol. 2007;74(2):116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldberg CS, Schwartz EM, Brunberg JA, Mosca RS, Bove EL, Schork MA, Stetz SP, Cheatham JP, Kulik TJ. Neurodevelopmental outcome of patients after the fontan operation: A comparison between children with hypoplastic left heart syndrome and other functional single ventricle lesions. J Pediatr. 2000;137(5):646–52. doi: 10.1067/mpd.2000.108952. [DOI] [PubMed] [Google Scholar]
- 12.Wheeler T, Gennser G, Lindvall R, Murrills AJ. Changes in the fetal heart rate associated with fetal breathing and fetal movement. Brit J. of Ob and Gyn. 1980;87:1068–1079. doi: 10.1111/j.1471-0528.1980.tb04475.x. [DOI] [PubMed] [Google Scholar]
- 13.Williams IA, Tarullo AR, Grieve PG, Wilpers A, Vignola EF, Myers MM, Fifer WP. Fetal cerebrovascular resistance and neonatal EEG predict 18-month neurodevelopmental outcomes in infants with congenital heart disease. Ultrasound in Ob & Gyn. 2012 Feb 20; doi: 10.1002/uog.11144. DOI:10.1002/uog.11144. [DOI] [PMC free article] [PubMed] [Google Scholar]