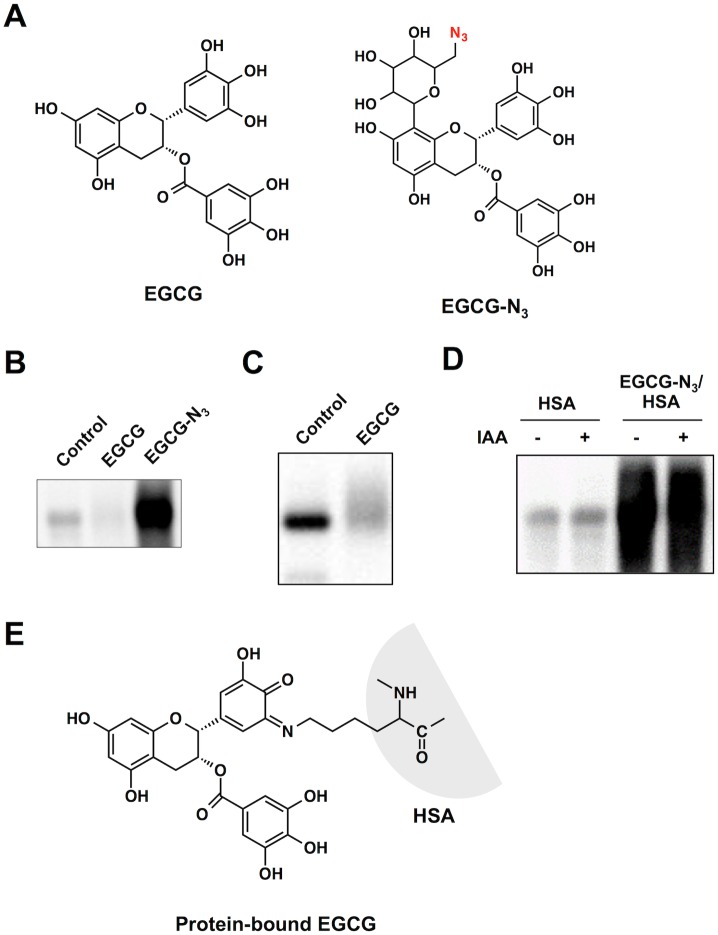
Fig 1. Analysis of a protein-bound EGCG using click chemistry.
(A) Chemical structures of EGCG and an azide-containing probe (EGCG-N3). (B) Fluorescent detection of protein-bound EGCG. HSA was pretreated with EGCG-N3 or vehicle for 24 h. The reaction mixtures were subjected to the click reaction with an alkynylated biotin followed by separation on SDS-PAGE gel. (C) Sulfhydryl modification of HSA by EGCG. NeutrAvidin blot analysis using iodoacetyl-LC-biotin. HSA (1 mg/ml) was incubated with EGCG (1 mM) in 0.1 ml of PBS (pH 7.4) for 24 h at 37°C. (D) Effect of IAA pre-treatment on the binding of EGCG-N3 to HSA. Native and IAA-treated HSA were incubated with and without EGCG-N3 and then subjected to the click chemistry. (E) A possible structure of an EGCG-lysine adduct in proteins.