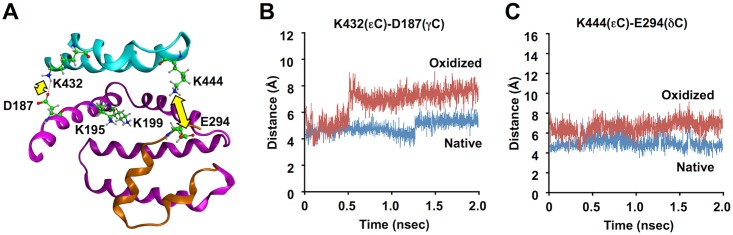
Fig 6. Changes in the electrostatic interaction of lysine residues as a consequence of oxidative deamination.
(A) Schematic representation of the EGCG binding site in subdomains IIA and IIIA. Electrostatic interactions between Lys-432 and Asp-187 and between Lys-444 and Glu-292 are shown with yellow arrows. (B) Time dependent evolution of distance between the Lys-432 ε-carbon and the carboxyl group (γ-COOH) of Asp-187 before and after AAS formation at Lys-432. (C) Time dependent evolution of distance between the Lys-444 ε-carbon to the carboxyl group (δ-COOH) of Glu-292 before and after AAS formation at Lys-444.