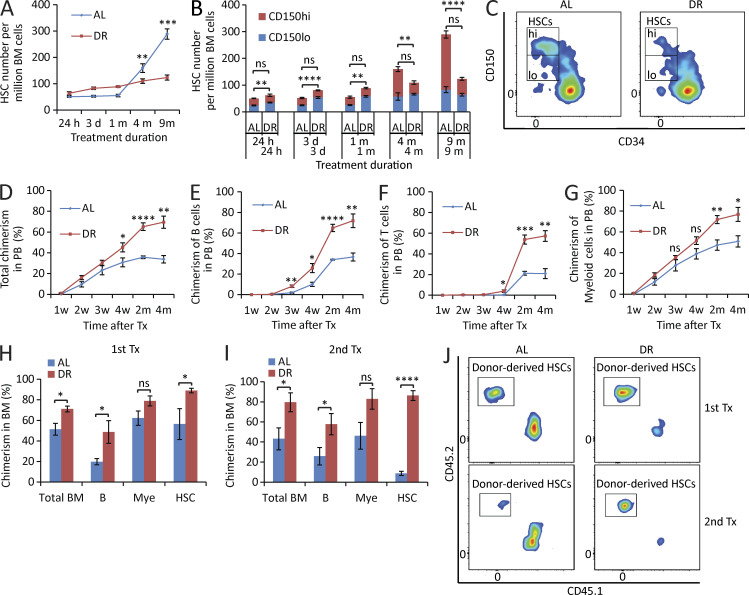
Figure 1.
DR retards HSC aging. (A–C) 3-mo-old mice were fed with DR diet or AL diet. The HSC population was analyzed by flow cytometry at the indicated time points after dietary intervention (n = 3–5 mice per group per time point; n = 2 independent experiments). Note that the number of HSCs, in particular myeloid-biased HSCs, of DR mice was maintained relatively stable, whereas it increased significantly over time during aging in AL mice. In B, the significance of the comparison was shown in the lower line for the lymphoid-biased HSCs (CD150lo HSCs) and in the upper line for the myeloid-biased HSCs (CD150hi HSCs). Note that the skewing toward myeloid-biased HSCs during aging in AL mice was rescued in DR mice. (C) Representative FACS plots of mice treated with 9-mo DR or AL gated from c-Kit+Sca-1+lineage− BM cells. (D–G) 100 HSCs derived from donor mice treated with mid-term (6 mo) DR or AL were transplanted along with 2 × 105 total BM cells from competitor mice into recipient mice (n = 4–5 mice per group; n = 2 independent experiments). Panels show donor-derived total chimerisms (D), chimerisms of lymphoid lineage (E and F), and chimerisms of myeloid lineage (G) in PB at the indicated time points after transplantation. (H–J) 200 HSCs derived from donor mice treated with long-term (1 yr) DR or AL were transplanted along with 2 × 105 total BM cells from competitor mice into recipient mice. 4 mo later, 107 BM cells from the primary recipients were transplanted to secondary recipient mice (n = 4–5 mice per group; n = 2 independent experiments). (H and I) Donor-derived chimerisms in BM 4 mo after primary (H) and secondary (I) transplantation (Tx). (J) Representative FACS plots of primary and secondary recipient mice gated from HSCs. HSCs, CD150+CD34−c-Kit+Sca-1+lineage− BM cells; m, months; B, B cells; T, T cells; Mye, myeloid cells; w, weeks. Data are displayed as mean ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001 by unpaired two-tailed Student’s t test. ns, not significant.