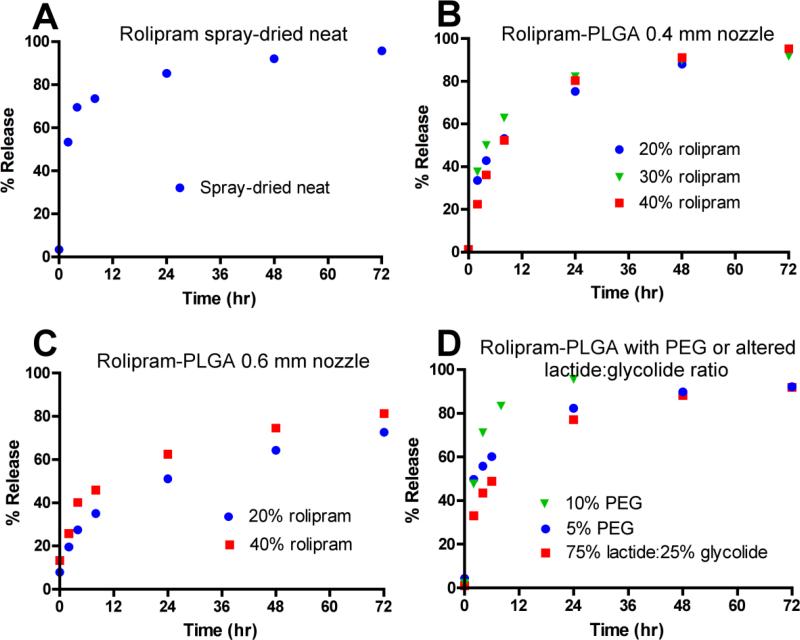
Figure 2. In vitro release kinetics for rolipram formulations.
Rolipram formulations produced by spray drying were evaluated using in vitro release assays. A) Neat rolipram, 0.4 mm nozzle. B) Rolipram/PLGA formulations containing 20%, 30%, and 40% rolipram, 0.4 mm nozzle. C) Rolipram/PLGA formulations containing 20% and 40% rolipram, 0.6 mm nozzle. D) Rolipram/PLGA formulations containing 40% rolipram with altered lactide:glycolide ratio or addition of PEG, 0.4 mm nozzle.