Abstract
Background
Developmental neurotoxicity of ketamine, an NMDA receptor antagonist, must be considered due to its widespread uses for sedation/analgesia/anesthesia in pediatric and obstetric settings. Dose-dependent effects of ketamine on cellular proliferation in the neurogenic regions of rat fetal cortex (ventricular zone (VZ) and subventricular zone (SVZ)) were investigated in this in vivo study.
Methods
Timed-pregnant Sprague-Dawley (SD) rats at embryonic day 17 (E17) were given with different doses of ketamine intraperitoneally (0, 1, 2, 10, 20, 40, and 100 mg kg-1). Proliferating cells in the rat fetal brains were labeled by injecting 100 mg kg-1 of 5-bromo-2′-deoxyuridine (BrdU) intraperitoneally. BrdU-labeled cells were detected by immunostaining methods. The numbers of BrdU positive cells in VZ and SVZ of rat fetal cortex were employed to quantify proliferation in the developing rat cortex.
Results
Ketamine dose-dependently reduced the number of BrdU positive cells in VZ (P<0.001) and SVZ (P<0.001) of the rat fetal cortex. SVZ showed greater susceptibility to ketamine-induced reduction of proliferation in rat fetal cortex, occurring even at clinically relevant doses (2 mg kg-1).
Conclusion
These data suggest that exposure to ketamine during embryogenesis can dose-dependently inhibit the cellular proliferation in neurogenic regions of the rat fetal cortex.
Keywords: Embryogenesis, ketamine, neural progenitor cells, neurogenesis, proliferation
Introduction
Ketamine, an NMDA (N-methyl-D-aspartate) receptor antagonist, is widely used as an anesthetic, analgesic, or sedative in obstetric and pediatric clinical practice. With increasing frequency, ketamine is also consumed as an illicit drug by pregnant drug abusers. Thus, fetuses of pregnant patients and drug abusers have an increasing incidence of exposure to ketamine. Recent studies have indicated the neurotoxic effects of ketamine on the developing brain, thus questioning the safety of ketamine in pediatric use.1-4 Most of these studies were focused on ketamine-induced cell death of newborn neurons in the postnatal developing brain.2,5-9 However, the potential detrimental effects of ketamine on neurogenesis in the developing fetal brain remain unclear.
NMDA antagonists (MK-801, phencyclidine, S(+)ketamine) were reported to alter the proliferation of neural progenitor cells in adult animal models.10-12 Previous studies indicated that NMDA receptor antagonists can inhibit neurogenesis in the hippocampus of injured brains, dose-dependently and age-dependently.11,12 Neurogenesis in the brain occurs throughout the entire lifespan, from early fetal to adult life, and can also be activated by exposure to ischemia, traumatic brain injury, or other insults. In the formative stages of brain development, the highest rates of neurogenesis occur in two specific regions, the ventricular zone (VZ) and subventricular zone (SVZ) of the fetal cortex.13 Most proliferating neural progenitor cells are born in VZ and SVZ, migrate, differentiate en route, become mature cells, and are incorporated into functional neural circuitry at their final endpoint. Normal proliferation in VZ and SVZ is one of the key factors, providing cellular elements for the development of normal brain neurocircuitry.
From in vitro cultures of neural stem/progenitor cells (NSPCs), we found that ketamine inhibits the proliferation and enhances the differentiation of neural progenitor cells.14 The aims of this study were to determine if ketamine changes the number of proliferating NSPCs in vivo in the rat fetal cortical neurogenic regions, and to examine whether these were dose-dependent or region-dependent effects.
Methods
Animals
Timed-pregnant Sprague-Dawley (SD) rats (N=14) were transported to our animal facilities on gestational/embryonic day 14 (E14) and housed at 24°C on a 12:12-hour light/dark cycles with free access to food and water until embryonic day 17 (E17) (rat pregnancy spans 21-23 days). Animals were randomly divided into 7 treatment groups. For each group, one randomly selected animal was exposed to different doses of ketamine or saline (0.9% NaCl w/v). Remaining batch of animals was used to independently duplicate the whole experiment. Body weights, fur and clinical appearances of all animals were monitored daily. All animal use procedures were approved by the IACUC (Institutional Animal Care and Use Committee) of the University of Tennessee Health Science Center (Approval number: AUP-8168R2, Nov. 1st, 2009) and complied with the ethical standards described in the NIH Guide for Laboratory Animals.
BrdU Labeling Protocol
Ketamine (KETASET® (Ketamine hydrochloride injection, USP, NDC 0856-2013-01, Pfizer Inc., Fort Dodge, USA) at different doses (0, 1, 2, 10, 20, 40, and 100 mg kg-1 body weight) was injected intraperitoneally into animals at E17. All treatments and procedures in the vehicle group are the same with other ketamine exposure groups. Procedures were performed gently and stressful stimuli to animals were avoided. After each injection, animals were observed for 30-60 minutes to ensure that no severe anesthetic complications occurred (especially respiratory depression). At 23.5 hours after ketamine injection, each rat was weighed for preparation of BrdU (5-bromo-2′-deoxyuridine) injection solutions. At 24 hours, 100 mg kg-1 body weight of BrdU was administrated intraperitoneally to each animal. At 24 hours after BrdU injections, pregnant rats were sacrificed using rapid asphyxiation in a CO2 chamber. Based on preliminary experiments, each pregnant rat can carry 12±3 (mean±SD) fetuses. Three embryonic brains were randomly collected from each pregnant animal assigned to a dosage group. Embryonic brains were fixed in cold freshly-made 4% paraformaldehyde (PFA) with overnight incubation at 4 °C followed by transfer to a cold cryopreservation solution of 20% sucrose for overnight dehydration at 4 °C. Cryopreserved embryonic brains were cold mounted in cryomedium, frozen at -16°C, and cut at 8°C into coronal sections (10 μM thick) using a cryostat machine.15 Brain sections were mounted onto gelatin pre-coated glass slides. Each slide held six sections with a total of 11∼14 slides obtained from each fetal brain.
BrdU immunostaining
Sections were immunostained with BrdU and DAPI (4′, 6-diamidino-2-phenylindole).16,17 Specifically, sections were washed in 0.1 M phosphate buffered saline (PBS) for three times, incubated in 1N hydrogen chloride (HCl) for 10 min and 2N HCl for 10 min at room temperature (RT), and placed into an incubator at 37°C for 20 min. The HCl was neutralized with 0.1 M borate buffer for 12 min at RT, followed by washes for three times using PBS.16 Goat serum (5%) incubation for 40 min at RT was used to block the nonspecific antigens. Samples were incubated with mouse anti-BrdU primary antibody (1:1000) at 4°C overnight, followed by rinses with PBS for 3 times. Sections were incubated in goat anti-mouse Alexa 488 secondary antibodies (1:1000) at RT for 1 hr. Then sections were incubated in 1 μg/ml DAPI for 10 min at RT to label all nuclei and rinsed with PBS for 3 times.17 Slides were mounted with glass coverslips using aqueous mounting medium. Images were captured using an Olympus BX60 upright fluorescent microscope with Hamamatsu imaging system (Hamamatsu C4742-95 camera and Imaging program-HCImage 2.1 Live Version).
Cell counting
A naïve person blinded to the whole experiment numbered the slides of the ketamine dose groups in a random order. A skilled technician also blinded to the numbering processes of ketamine dose groups was responsible for counting the BrdU-positive and DAPI-positive cells. Among all slides from one embryonic brain (about 11∼14 slides), the first slide selected for image capture was the slide on which the brain section contains apparent lateral ventricle. Then, six continuous stained slides were selected for further analysis. On each selected slide, only the third and sixth tissue sections (for a total of 12 sections per embryonic brain) were selected for the final quantitative analysis. For each section, we captured images for three specified cortex regions lining on the dorsolateral side of the lateral ventricle (illustrated in Fig. 1A; A, B, and C squares represent the positions of the view fields on the dorsolateral cortex) at 400× magnification under a fluorescence microscope (Olympus, Japan). Each captured image contained a part of the lateral ventricle, ensuring that the thickness of the lateral ventricular wall was more than 50 μm.
Fig. 1.
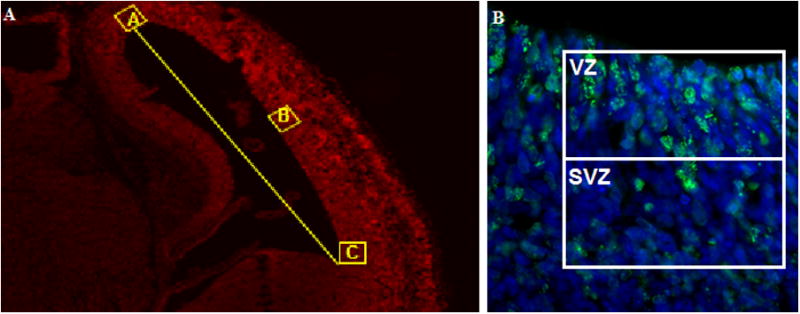
Sketch graphs showing cell counting strategy. On each stained brain section, three images were capatued for further statistical analysis. Fig. 1A, the three image capturing windows are showed as squares A, B, and C; The three square windows are selected at the dorsal lateral cortex; Square B is at the middle of the connecting line between A and C; Each square window is required to include one small part of the lateral ventricle. Fig. 1B, designed counting windows for ventricular zone (VZ) and subventricular zone (SVZ) in imaging analysis. On each image, a 50μm × 50μm square was drawn as the counting window and one side of the square was lined by the wall of lateral ventricle. A level bar was added into the square parallel to the lateral ventricular wall, which separated the square evenly into VZ and SVZ.
On each image, a 50μm × 50μm square was drawn as the counting window and one side of the square was lined by the wall of lateral ventricle.18 A level bar was added into the square parallel to the lateral ventricular wall, which separated the square evenly into two parts. The half next to the lateral ventricles was considered as VZ counting window, while the other half was SVZ counting window. (Fig. 1B) On each image, the numbers of BrdU-positive cells within the two windows were counted (for VZ and SVZ respectively). A sum of the numbers of BrdU-positive cells in VZ and SVZ counting windows represented the proliferation of the selected brain region. Based on 12 sections per animal, 3 regions per section, and two windows per region, cell counting was performed in 12×3×2=72 windows for each brain.
Statistical analysis
The numbers of BrdU-positive cells of all selected sections from one embryonic brain were summed as the total number of proliferating cells in that brain. The sum of BrdU positive cells in all VZ (or SVZ) windows for one brain represented the proliferation activity in VZ (or SVZ) of that brain. For each ketamine dose, we counted cells in 6 embryonic brains from two independently duplicated experiments for the quantitative analysis (n=6).
Based on previous in vitro studies, ketamine reduced the proportion of proliferating cells from 45±1.6% to 32.3±1.4%.14 Using α = 0.05 and 1–β = 0.90, a power analysis for comparing 7 groups via ANOVA was performed, giving us a sample size N=5 per group. We included 6 animals per group, to allow for unanticipated contingencies.19 All statistical data are shown as Mean±SD (Mean±standard deviation). One-way analysis of variance (ANOVA) followed by post hoc Bonferroni tests was performed to determine whether significant changes were due to different ketamine doses. F-test for two sample variance was performed between each ketamine dose group and the placebo control (0 mg kg-1) group to assess whether the variances of the two groups were equal. If the two variances were equal, a t-test for two samples assuming equal variances was performed to compare the means of those two groups. If not, a t-test for two samples assuming unequal variances was performed for comparison of the two groups. In all statistical analyses, critical significance level was set at P<0.05. GraphPad3 and Microsoft Office Excel were utilized for these statistical analyses.
Results
Seven groups of timed-pregnant SD rats were injected intraperitoneally with different doses of ketamine (0, 1, 2, 10, 20, 40, and 100 mg kg-1). Proliferating cells in the fetal brain were labeled using BrdU and visualized by immunostaining methods. The pseudostratified ventricular epithelium containing VZ and SVZ showed the largest number of BrdU-positive cells (Fig. 1B).
After serial imaging, cell counting, and statistical analysis, total numbers of BrdU-positive cells counted for each dose are shown as Mean±SD (Table 1, Figures 2A and 3). Compared to the vehicle group (0 mg kg-1), high doses of ketamine (20, 40, and 100 mg kg-1, more than an anesthetic dose 10 mg kg-1) resulted in significant decreases of BrdU-positive cells in the developing neocortex of embryonic brains. Lower doses of ketamine (1, 2, or 10 mg kg-1) did not induce apparent changes in BrdU-positive cells of the fetal neocortex. These data indicate that high doses of ketamine (≥20 mg kg-1) dose-dependently inhibit the proliferation in neurogenic regions of the fetal neocortex at E17-E19.
Table 1.
Statistical data in detecting the dose dependent effects of ketamine on the proliferation of the cortex of the developing brain using BrdU incorporation assay.
| Dose (mg kg-1) | vehicle | 1 | 2 | 10 | 20 | 40 | 100 |
|---|---|---|---|---|---|---|---|
| Total | 2860 ± 175 n=6 |
2744 ± 102 n=6 P=0.18 |
2705 ± 125 n=6 P=0.11 |
2690 ± 82 n=6 P=0.055 |
2586 ± 122 n=6 P=0.0004 |
2426 ± 107 n=6 P=0.0004 |
2136 ± 130 n=6 P<0.001 |
| VZ | 1569 ± 93 n=6 |
1532 ± 86 n=6 P=0.50 |
1531 ± 56 n=6 P=0.41 |
1550 ± 63 n=6 P=0.68 |
1431 ± 63 n=6 P=0.013 |
1365 ± 41 n=6 P=0.0006 |
1220 ± 58 n=6 P<0.001 |
| SVZ | 1291 ± 88 n=6 |
1212 ± 56 n=6 P=0.092 |
1174 ± 91 n=6 P=0.046 |
1140 ± 52 n=6 P=0.0047 |
1155 ± 77 n=6 P=0.016 |
1061 ± 91 n=6 P=0.0012 |
916 ± 88 n=6 P<0.001 |
Notes: data are shown as mean ± SD. Six embryonic brains in each ketamine dose group were used for the statistical analysis (n=6). P-values are calculated using t-test by comparing with the vehicle group. Significant difference is set at P < 0.05. Bolded numbers in the table indicate the significant difference compared with vehicle group data. Total: the sum of the numbers of BrdU positive cells at VZ and SVZ.
Fig. 2.
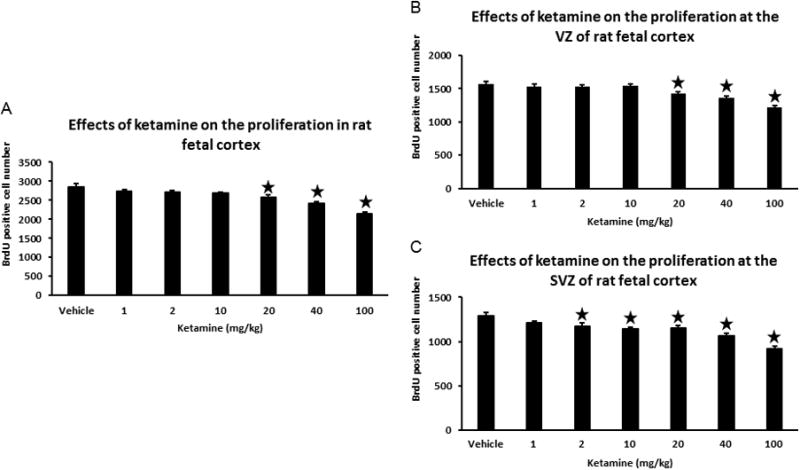
Ketamine inhibits the proliferation at VZ and SVZ of rat embryonic cortex. Fig. 2A, changes of total BrdU positive cells at VZ and SVZ of embryonic cortex at the exposure to different doses of ketamine. Fig. 2B, effects of ketamine on the proliferation in VZ of rat embryonic cortex, the changes of BrdU positive cells at VZ of embryonic cortex under the exposure to different doses of ketamine. Fig. 2C, effects of ketamine on the proliferation in SVZ of rat embryonic cortex, the changes of BrdU positive cells at SVZ of embryonic cortex under the exposure to different dose of ketamine. One-way ANOVA is performed to determine significant difference in the experiments (P<0.001). All data are shown as Mean±SD, bars present standard deviation. Significant difference was set at P<0.05. Stars represent the significant difference by comparing with the vehicle.
Fig. 3.
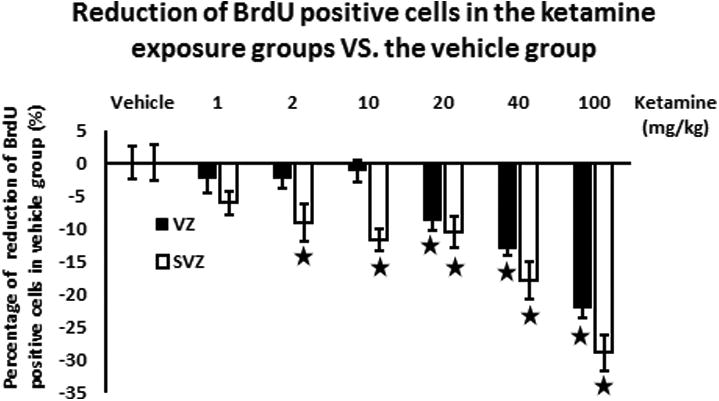
Reduction (%) of BrdU positive cells compared to the vehicle group in the different ketamine exposure groups. Reduction of proliferation in the ketamine exposure groups compared to the vehicle group. Black solid column represents the ventricular zone while the white column shows the subventricular zone. For the ventricular zone, a significant decrease of BrdU positive cells was found in the experimental groups exposed to ketamine ≥ 20 mg kg-1. For the subventricular zone, a significant reduction of BrdU positive cells appeared in the ketamine exposure groups (≥ 2 mg kg-1) and the reduction is in a dose-dependent ways. In the 100 mg kg-1ketamine group, decreases of BrdU positive cells were induced in VZ and SVZ of rat fetal cortices.
Further analyses were performed to examine dose-dependent effects of ketamine on proliferating cells in VZ and SVZ (Table 1). Among high-dose ketamine groups (20, 40, and 100 mg kg-1), the number of BrdU-positive cells in VZ decreased significantly. This declining trend was similar to the total number of BrdU-positive cells (Fig. 2B and 3). Smaller doses of ketamine (1, 2, or 10 mg kg-1) did not change the number of BrdU-positive cells in VZ. In SVZ, however, the numbers of BrdU-positive cells were reduced even in lower-dose ketamine groups, with significant decreases occurring in 2 mg kg-1 ketamine group compared to the vehicle (P=0.046). Higher ketamine doses (10, 20, 40, and 100 mg kg-1) also led to decreasing numbers of BrdU-positive cells in SVZ (Fig. 2C and 3). Thus, even clinically relevant doses of ketamine (2, 10 mg kg-1) may decrease of proliferation in SVZ.
Combining the results of VZ and SVZ, the numbers of BrdU-positive cells in VZ and SVZ decreased in a dose-dependent manner (Fig. 3). The reduction percentage of BrdU-positive cells in SVZ exposed to ketamine compared to the vehicle group showed a decreasing trend along with the increase of ketamine injection dose (Fig. 3). This implies that NSPCs in SVZ may be more sensitive to anti-proliferative effects of ketamine. Taken together, these in vivo study results suggest that high doses of ketamine (≥20 mg kg-1) can inhibit the proliferation in VZ and SVZ of the developing cortex of embryonic brains at E17-19 in a dose-dependent manner.
Discussion
We previously reported that prolonged exposure (48 hours) or high concentrations (20-100μM) of ketamine significantly inhibited proliferation of NSPCs cultured in vitro, and that ketamine exposure enhanced neuronal differentiation at all tested concentrations (1-100μM for 24 hours) and at clinically relevant concentrations (10μM) for 10 hours or longer.14 This in vivo study shows that ketamine dose-dependently reduces BrdU-positive cells in VZ and SVZ of embryonic brains exposed to high doses of ketamine (20, 40, and 100 mg kg-1). This in vivo result not only confirms our previous in vitro studies, but also is consistent with the results of other published studies, exploring ketamine effects in different neurogenic brain regions (SGZ) and in normal or pathological brains.12,20-25 Therefore, we propose that ketamine can inhibit the proliferation of NSPCs in the neocortex of embryonic brains at E17-19.
The timing of exposure of ketamine to rat embryonic brain at embryonic Day 17∼19 could be equivalent to the early development stage of human brain. A neuroinformatics model for translating brain development timing across mammalian species26 suggests that neurogenesis in the rat cortex at post-conception day 19 translates to human post-conception day 123. Thus, our results on ketamine effects on neurogenesis in the rat neocortex correspond to the second trimester of human gestation.
In this study, BrdU incorporation assays are effective to detect changes in proliferation in the developing brain exposed to different doses of ketamine. Although Ki-67 immuno-staining methods are alternatives to detect proliferative cells at G1, S, G2, and M phases of cell cycles, practically, more than 90% of Ki-67 positive cells in VZ and SVZ of the developing brain increase the difficulty in accurate cell counting. Based on these considerations, BrdU incorporation assays are more appropriate methods for this study assessing the number of proliferating cells in rat fetal neocortices.
Proliferating cells in SVZ were more susceptible to ketamine-induced inhibition than those in VZ (Fig. 2 and 3). The majority of undifferentiated neural stem cells (NSCs) are located in VZ, which is the “proliferation engine”, producing large numbers of NSCs and neural progenitor cells (NPCs) in the developing brain. After new NPCs are generated in VZ, they migrate toward the outer cortical layers. Thus, large numbers of NPCs are located in SVZ, and are more differentiated than NSCs in VZ. Previous in vitro study showed that ketamine enhances differentiation of NSPCs in a dose- and time-dependent manner.14 Thus, a single dose of ketamine may induce NPCs in SVZ to differentiate into more mature NPCs or neurons which are progressively losing proliferative potency, whereas NSCs in VZ just may be induced to become NPCs while still possessing the capacity for proliferation. This may explain the greater reduction of BrdU-positive cells in SVZ than in VZ following exposure to a high dose of ketamine.
Previous studies have already implicated that some intracellular mechanisms may be involved in ketamine-induced inhibitory effects on proliferation in the brain growth and the kidney development. During the brain growth spurt period peaking around rat postnatal day 10, ketamine altered the biochemical substrates for neuronal growth and synaptogenesis, such as calcium/calmodulin-dependent protein kinase II (CaMKII) and growth associated protein-43 (GAP-43).27 The inhibitory effects of ketamine on de novo proliferation were reported in cultured rat mesangial cells (MCs) associating with ketamine changed the levels of TNF-α, IL1-, IL6-, and angiotensin II in MCs.28 Our recent study indicate that the PI3K (phosphatidylinositide 3-kinases)/Akt-p27 signaling pathway plays a role in ketamine inhibited proliferation in neural stem progenitor cells in the developing rat brain.29
Single moderate doses of ketamine (5, 10, or 25 mg kg-1 injected subcutaneously) significantly altered these substrates, associated with functional deficits in spontaneous behaviors and habituation persisting into adulthood.27 Our report documents significant inhibitory effects of ketamine exposure during embryogenesis on proliferation in rat fetal cortical neurogenic regions in vivo. A recent study suggested that maternal anesthesia with ketamine during the fetal rat brain development reduces proliferation in hippocampus of offspring, induces subsequent neurobehavioral abnormalities such as depression- and anxiety-like behaviors, and impairs memory in young adults.30 However, mechanisms and long-term consequences of ketamine-induced proliferation inhibition remain unclear.
Concerning limitations, it cannot be definitively excluded that ketamine-induced changes in maternal systemic physiology (heart rate, blood pressure, body temperature) had any influence on embryonic brains. High doses of ketamine (50-100 mg kg-1) can lead to severe respiratory depression and death. In preliminary experiments, ketamine 100 mg kg-1 injected intraperitoneally to one pregnant rat caused severe respiratory depression and death, whereas smaller doses were well tolerated. Thus, in the reported study the 100 mg kg-1 ketamine dose was divided equally (50 mg kg-1 each) and injected intraperitoneally with a 5-minute interval to reduce the incidence of respiratory depression. All animals were carefully observed color, movement, and respiratory rate for a minimum of 30 minutes after ketamine injection. Apparent changes in color or respiratory rate did not occur in these experiments, but undefined effects of ketamine on the maternal systemic physiology could indirectly affect the proliferation of embryonic NSPCs.
The experiments were independently completed twice and three embryos of each pregnant animal were collected for each treatment group (n=6). Based on the sample size (n=6), a post hoc calculated power of 0.88, which corresponds to the 1–β-error assumed at the design of our experiments and supports the results of this study. The consistence of the results from those independently duplicated experiments reflects the reproducibility of this study.
Ketamine does induce apoptosis in mature neurons, as reported previously7. Ketamine has been identified as a neurotoxin in rodent animal models due to cell death induced in developing neurons. However, previous in vitro studies showed that NSPCs were remarkably resistant to neurotoxic effects of ketamine, with no increases in apoptosis or necrosis in cultured NSPCs.14 Assuming that most cells in neurogenic regions are proliferative NSPCs which are resistant to ketamine-induced cell death, ketamine could not induce statistically significant cell death in neurogenic regions.
In conclusion, the exposure to ketamine during embryogenesis can dose-dependently inhibit the cellular proliferation in neurogenic regions of the rat fetal cortex. The finding implicates a large dose of ketamine could reduce the quantity of neurons at early developmental stages of the fetal cortex, which may induce potential negative behavioral and cognitive consequences across the lifespan. Currently, ketamine is widely used as an anesthetic, analgesic, and sedative in pediatric and obstetric settings and also is an illicit club drug consumed by increasing numbers of pregnant drug abusers. Since previous animal studies indicated potential negative effects of ketamine on the developing brain, more animal experiments and relevant clinical trials should be focused on identifying the developmental neurotoxicity of ketamine and evaluating the safety of its clinical uses.
Acknowledgments
Funding: Supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) via U10-HD50009, National Natural Science Foundation of China via No. 81503167, the Arkansas Children's Hospital Student and Clinical Staff Research Grant, and the Oxnard Foundation.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest.
Editorial Comment: There is concern that some anesthetics, including ketamine, may be neurotoxic in the developing brain. In this experimental study in a rat fetal model, ketamine exposure was associated with reduced proliferation of cortical cells, and this in a dose dependent manner, as a sign of possible neurotoxicity in this model.
References
- 1.Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K, Tenkova TI, Stefovska V, Turski L, Olney JW. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science. 1999;283:70–4. doi: 10.1126/science.283.5398.70. [DOI] [PubMed] [Google Scholar]
- 2.Zou X, Patterson TA, Divine RL, Sadovova N, Zhang X, Hanig JP, Paule MG, Slikker W, Jr, Wang C. Prolonged exposure to ketamine increases neurodegeneration in the developing monkey brain. Int J Dev Neurosci. 2009;27:727–31. doi: 10.1016/j.ijdevneu.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Slikker W, Jr, Zou X, Hotchkiss CE, Divine RL, Sadovova N, Twaddle NC, Doerge DR, Scallet AC, Patterson TA, Hanig JP, Paule MG, Wang C. Ketamine-induced neuronal cell death in the perinatal rhesus monkey. Toxicol Sci. 2007;98:145–58. doi: 10.1093/toxsci/kfm084. [DOI] [PubMed] [Google Scholar]
- 4.Dong C, Anand KJS. Developmental neurotoxicity of ketamine in pediatric clinical use. Toxicology letters. 2013;220:53–60. doi: 10.1016/j.toxlet.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 5.Takadera T, Ishida A, Ohyashiki T. Ketamine-induced apoptosis in cultured rat cortical neurons. Toxicol Appl Pharmacol. 2006;210:100–7. doi: 10.1016/j.taap.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Soriano SG, Liu Q, Li J, Liu JR, Han XH, Kanter JL, Bajic D, Ibla JC. Ketamine activates cell cycle signaling and apoptosis in the neonatal rat brain. Anesthesiology. 2010;112:1155–63. doi: 10.1097/ALN.0b013e3181d3e0c2. [DOI] [PubMed] [Google Scholar]
- 7.Wang C, Sadovova N, Fu X, Schmued L, Scallet A, Hanig J, Slikker W. The role of the N-methyl-D-aspartate receptor in ketamine-induced apoptosis in rat forebrain culture. Neuroscience. 2005;132:967–77. doi: 10.1016/j.neuroscience.2005.01.053. [DOI] [PubMed] [Google Scholar]
- 8.Wang C, Sadovova N, Hotchkiss C, Fu X, Scallet AC, Patterson TA, Hanig J, Paule MG, Slikker W., Jr Blockade of N-methyl-D-aspartate receptors by ketamine produces loss of postnatal day 3 monkey frontal cortical neurons in culture. Toxicol Sci. 2006;91:192–201. doi: 10.1093/toxsci/kfj144. [DOI] [PubMed] [Google Scholar]
- 9.Zou X, Patterson TA, Sadovova N, Twaddle NC, Doerge DR, Zhang X, Fu X, Hanig JP, Paule MG, Slikker W, Wang C. Potential neurotoxicity of ketamine in the developing rat brain. Toxicol Sci. 2009;108:149–58. doi: 10.1093/toxsci/kfn270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mochizuki N, Takagi N, Kurokawa K, Kawai T, Besshoh S, Tanonaka K, Takeo S. Effect of NMDA receptor antagonist on proliferation of neurospheres from embryonic brain. Neurosci Lett. 2007;417:143–8. doi: 10.1016/j.neulet.2007.02.066. [DOI] [PubMed] [Google Scholar]
- 11.Tung A, Herrera S, Fornal CA, Jacobs BL. The effect of prolonged anesthesia with isoflurane, propofol, dexmedetomidine, or ketamine on neural cell proliferation in the adult rat. Anesth Analg. 2008;106:1772–7. doi: 10.1213/ane.0b013e31816f2004. [DOI] [PubMed] [Google Scholar]
- 12.Winkelheide U, Lasarzik I, Kaeppel B, Winkler J, Werner C, Kochs E, Engelhard K. Dose-dependent effect of S(+) ketamine on post-ischemic endogenous neurogenesis in rats. Acta Anaesthesiol Scand. 2009;53:528–33. doi: 10.1111/j.1399-6576.2009.01905.x. [DOI] [PubMed] [Google Scholar]
- 13.Bystron I, Blakemore C, Rakic P. Development of the human cerebral cortex: Boulder Committee revisited. Nat Rev Neurosci. 2008;9:110–22. doi: 10.1038/nrn2252. [DOI] [PubMed] [Google Scholar]
- 14.Dong C, Rovnaghi CR, Anand KJS. Ketamine alters the neurogenesis of rat cortical neural stem progenitor cells. Crit Care Med. 2012;40:2407–16. doi: 10.1097/CCM.0b013e318253563c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Emsley JG, Hagg T. Endogenous and exogenous ciliary neurotrophic factor enhances forebrain neurogenesis in adult mice. Exp Neurol. 2003;183:298–310. doi: 10.1016/s0014-4886(03)00129-8. [DOI] [PubMed] [Google Scholar]
- 16.Li L, Walker TL, Zhang Y, Mackay EW, Bartlett PF. Endogenous interferon gamma directly regulates neural precursors in the non-inflammatory brain. J Neurosci. 2010;30:9038–50. doi: 10.1523/JNEUROSCI.5691-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eom TY, Jope RS. Blocked inhibitory serine-phosphorylation of glycogen synthase kinase-3alpha/beta impairs in vivo neural precursor cell proliferation. Biol Psychiatry. 2009;66:494–502. doi: 10.1016/j.biopsych.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nasrallah HA, Hopkins T, Pixley SK. Differential effects of antipsychotic and antidepressant drugs on neurogenic regions in rats. Brain Res. 2010 doi: 10.1016/j.brainres.2010.07.075. [DOI] [PubMed] [Google Scholar]
- 19.Lenth RV. Java Applets for Power and Sample Size. [Accessed Oct. 6, 2015];2006-9 at http://www.stat.uiowa.edu/∼rlenth/Power)
- 20.Arvidsson A, Kokaia Z, Lindvall O. N-methyl-D-aspartate receptor-mediated increase of neurogenesis in adult rat dentate gyrus following stroke. Eur J Neurosci. 2001;14:10–8. doi: 10.1046/j.0953-816x.2001.01611.x. [DOI] [PubMed] [Google Scholar]
- 21.Bernabeu R, Sharp FR. NMDA and AMPA/kainate glutamate receptors modulate dentate neurogenesis and CA3 synapsin-I in normal and ischemic hippocampus. J Cereb Blood Flow Metab. 2000;20:1669–80. doi: 10.1097/00004647-200012000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Wang CZ, Yang SF, Xia Y, Johnson KM. Postnatal phencyclidine administration selectively reduces adult cortical parvalbumin-containing interneurons. Neuropsychopharmacology. 2008;33:2442–55. doi: 10.1038/sj.npp.1301647. [DOI] [PubMed] [Google Scholar]
- 23.Shen L, Zhang J. NMDA receptor and iNOS are involved in the effects of ginsenoside Rg1 on hippocampal neurogenesis in ischemic gerbils. Neurol Res. 2007;29:270–3. doi: 10.1179/016164107X159144. [DOI] [PubMed] [Google Scholar]
- 24.Huang H, Liu L, Li B, Zhao PP, Xu CM, Zhu YZ, Zhou CH, Wu YQ. Ketamine interferes with the proliferation and differentiation of neural stem cells in the subventricular zone of neonatal rats. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2015;35:315–25. doi: 10.1159/000369698. [DOI] [PubMed] [Google Scholar]
- 25.Wu YQ, Liang T, Huang H, Zhu YZ, Zhao PP, Xu CM, Liu L, Shi XT, Hu Y, Huang L, Zhou CH. Ketamine inhibits proliferation of neural stem cell from neonatal rat hippocampus in vitro. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2014;34:1792–801. doi: 10.1159/000366379. [DOI] [PubMed] [Google Scholar]
- 26.Workman AD, Charvet CJ, Clancy B, Darlington RB, Finlay BL. Modeling transformations of neurodevelopmental sequences across mammalian species. J Neurosci. 2013;33:7368–83. doi: 10.1523/JNEUROSCI.5746-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Viberg H, Ponten E, Eriksson P, Gordh T, Fredriksson A. Neonatal ketamine exposure results in changes in biochemical substrates of neuronal growth and synaptogenesis, and alters adult behavior irreversibly. Toxicology. 2008;249:153–9. doi: 10.1016/j.tox.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 28.Jimi N, Segawa K, Minami K, Sata T, Shigematsu A. Inhibitory effect of the intravenous anesthetic, ketamine, on rat mesangial cell proliferation. Anesth Analg. 1997;84:190–5. doi: 10.1097/00000539-199701000-00034. [DOI] [PubMed] [Google Scholar]
- 29.Dong C, Rovnaghi CR, Anand KJS. Ketamine affects the neurogenesis of rat fetal neural stem progenitor cells via the PI3K/Akt-p27 signaling pathway. Birth defects research Part B, Developmental and reproductive toxicology. 2014;101:355–63. doi: 10.1002/bdrb.21119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao T, Li Y, Wei W, Savage S, Zhou L, Ma D. Ketamine administered to pregnant rats in the second trimester causes long-lasting behavioral disorders in offspring. Neurobiology of disease. 2014;68:145–55. doi: 10.1016/j.nbd.2014.02.009. [DOI] [PubMed] [Google Scholar]


