Figure 1. Adoptive transfer of BCL2+IL6+ B cells gives rise to plasma cell neoplasms (PCN) in host mice.
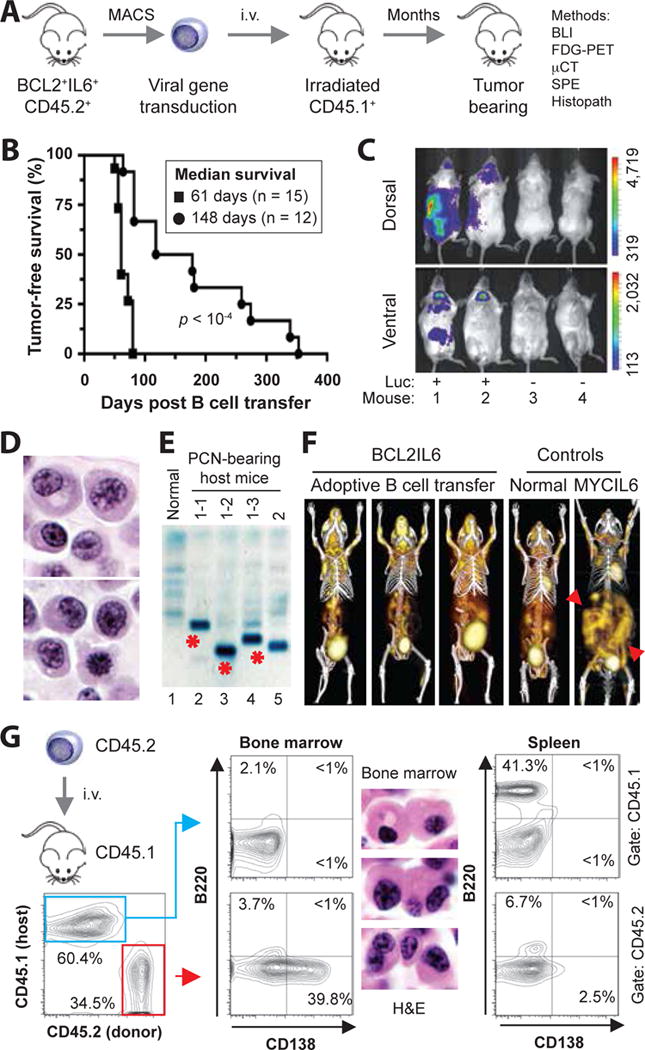
(A) Schematic overview of tumor induction and analysis. The experimental approach relied on the adoptive transfer of B220+ B cells obtained from spleens of 5–8 weeks old, double-transgenic BCL2+IL6+ mice congenic for CD45.2+. Donor B cells were isolated with the assistance of the MACS® B220 mouse B-cell kit from Miltenyi, were in some cases genetically modified in vitro by retroviral reporter gene (luciferase) transduction, and were transferred to normal (not transgenic) CD45.1+ host mice that had been conditioned with whole-body irradiation a few hours prior to the cell transfer. Neoplasms arising in B cell-reconstituted hosts were analyzed using a variety of immunological, imaging and histopathological methods, the results of which are presented in panels B to G of this figure and Figure 2. Note that one donor mouse suffices to reconstitute 20–30 hosts, which mimic the clonal heterogeneity of human myeloma [4] by developing distinct tumors. See panel E for an example. Donor B cells at the time of harvest can be operationally defined as premalignant. However, their malignant potential is high, as all BCL2+IL6+ mice spontaneously develop striking hypergammaglobulinemia by ~3 months of age (Supplemental Figure 6) and PC tumors by ~5 months of age (Supplemental Figure 7).
(B) Line graphs indicating tumor-free survival of B-cell reconstituted host mice pre-treated with either a sublethal dose (4.5 Gy, black circles) or lethal dose (11 Gy, black squares) of whole-body irradiation. The latter was administered as split dose (4.5 Gy and 6.5 Gy, delivered 4 hrs apart) and required hematopoietic stem cell rescue using bone marrow transplantation (3 × 106 cells) for survival of mice. Median tumor-free survival of sublethally irradiated hosts (148 days; n=12) was significantly longer than lethally irradiated hosts (61 days; n = 15) based on Mantel-Cox log-rank analysis (p < 10−4). In both studies, 2 × 106 donor B cells were transferred. B cells were stimulated for about 48 hrs in vitro using 12.5 ng/ml mIL-4 and 10 μg/ml LPS (both from Sigma, St. Louis, MO).
(C) Bioluminescence image of 4 B cell-reconstituted, tumor-bearing CD45.1+ recipients that had received on day -76 either luciferase-expressing CD45.2+B220+ B cells (Luc+) or B cells not transduced with luciferase-encoding cDNA (Luc−). The result indicates that tumor precursors may be genetically modified in future studies by retroviral transduction of over-expressed candidate myeloma driver genes or shRNAs knocking down those genes.
(D) High-power view (100×) of H&E-stained PC tumor tissue section demonstrating typical features of malignant PCs, such as large amounts of cytoplasm, eccentric nucleus with marginated chromatin and paranuclear hof.
(E) Serum protein electropherogram demonstrating pronounced M-spikes (monoclonal Ig) in 4 PCN-bearing CD45.1+ mice reconstituted with B cells from the same donor mouse (lanes 2–4) or a different donor mouse (lane 5). Normal serum (lane 1) was used as control. The different electrophoretic mobility of the paraproteins in lanes 2–4 (red asterisks) strongly suggests that the underlying PC tumors are unique; i.e., different tumor precursors from the donor B cell pool underwent tumor progression and attained clonal dominance. Additional changes of serum proteins in tumor-bearing mice included elevated levels of cyto- and chemokines (e.g., IL-5, IL-13, IL-17, MCP-1 and TNFα) relative to tumor-bearing IL6MYC mice [8] and BCL2+IL6+ mice (Supplemental Tables 1 and 2, Supplemental Figure 8).
(F) Three dimensional renderings of fused FDG-PET/CT images obtained from three adoptively transferred mice that carried BCL2+IL6+ PC tumors (images 1–3), an age-matched normal C mouse used as control (image 4) and a PC tumor-bearing MYCIL6-transgenic mouse (image 5) that exhibited pronounced extraosseous disease in abdominal lymph nodes (red arrowhead pointing down) and spleen (red arrowhead pointing up). All images were normalized to the same maximal standard uptake value (SUVmax) to facilitate comparison of PET lesions. High FDG uptake and PET signal strength not associated with tumor burden is apparent in tissues with physiologically high levels of glucose utilization (e.g., brain, heart) and in the FDG excretion pathway, particularly the urinary bladder.
(G) Flow cytometric histograms of a representative CD45.2+ myeloma-like tumor that resides in a CD45.1+ host. Forward scatter- and side scatter-gated bone marrow cells in the contour plot to the left were first distinguished as donor derived (CD45.2+, 34.5%) or host derived (CD45.1+, 60.4%) and then evaluated for the expression of B220 (B cell marker) and CD138 (syndecan-1, plasma cell marker). Unlike host cells, which included a small number of B220+CD138− B cells (2.1%) but essentially no B220+CD138+ plasmablasts (<1%) or B220−CD138+ PCs (<1%; center plot, top) in the bone marrow, donor cells contained a sizable fraction of PCs (39.8%; center plot, bottom). These cells exhibited the hallmark cytological features of myeloma in H&E-stained tissue sections (original magnification 100×). The spleen of the same mouse harbored a large number of host-derived B cells (41.3%; right plot, top), as one might have expected in a situation in which the infiltration with donor-derived B cells (6.7%) and plasma cells (2.5%) is just beginning. The low percentage of malignant PCs in the spleen underlines the bone-seeking, myeloma-like homing pattern of the adoptive-transfer tumors presented in panel F.
