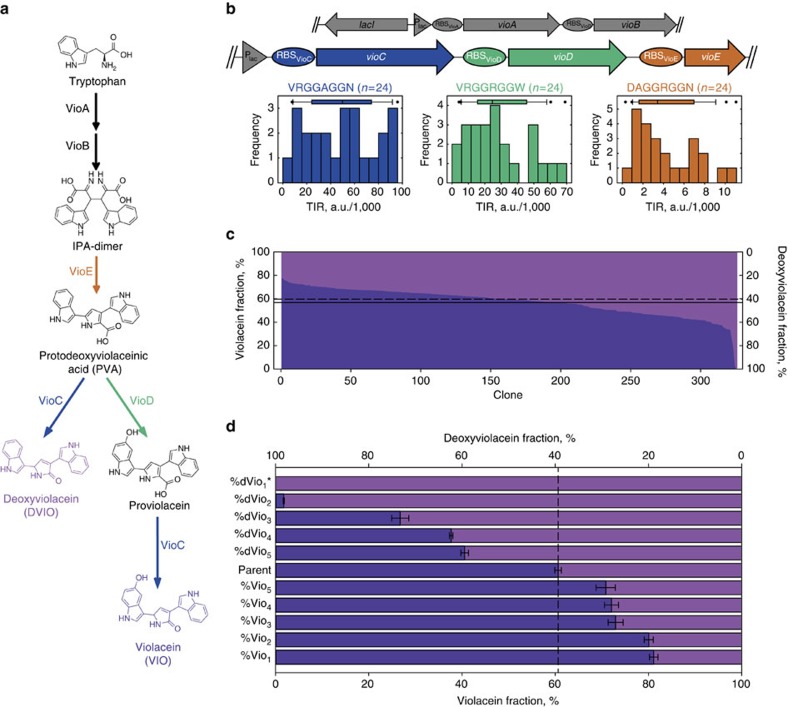
Figure 4. Rationally reduced library for optimization of violacein production in E. coli.
(a) The pigment violacein (VIO) is produced in five heterologous reactions (catalysed by VioABEDC) from L-tryptophan. The branched nature of the pathway leads to the formation of other pigments including the main byproduct deoxyviolacein (DVIO). (b) For pathway optimization the genes vioC, vioD and vioE were organized in an operon-like structure (plasmid pMJ3). The expression levels were uniformly randomized by implementing a rationally reduced library with a target size of 24 produced by RedLibs out of an initial N8 data set for each of the respective proteins. VioA and B were synthesized from a helper plasmid (pMJ2). (c) The rationally reduced RBS library allowed for the creation of a phenotypic variety of clones with distinctly altered product spectra as compared to the library average (solid line) and to the parent clone (pMJ3 with unaltered RBSs, dashed line). With a limited screening effort (372 clones in 96-well microtiter plates) mutants with significantly altered selectivity for the production of VIO and DVIO could be obtained. Please note that of the 372 clones analysed 47 were excluded from the figure because they did not produce detectable amounts of either pigment. (d) Quantitative pigment extraction of the five clones with the highest selectivity for either VIO (%Vio1–5) or DVIO (%dVio1–5). Data represents the mean of four independent replicate cultures with standard deviation. *Please note that clone %dVio1 had an extra stop codon inserted in vioD and can therefore be considered a knockout mutant for this gene leading to pure DVIO production (compare a).