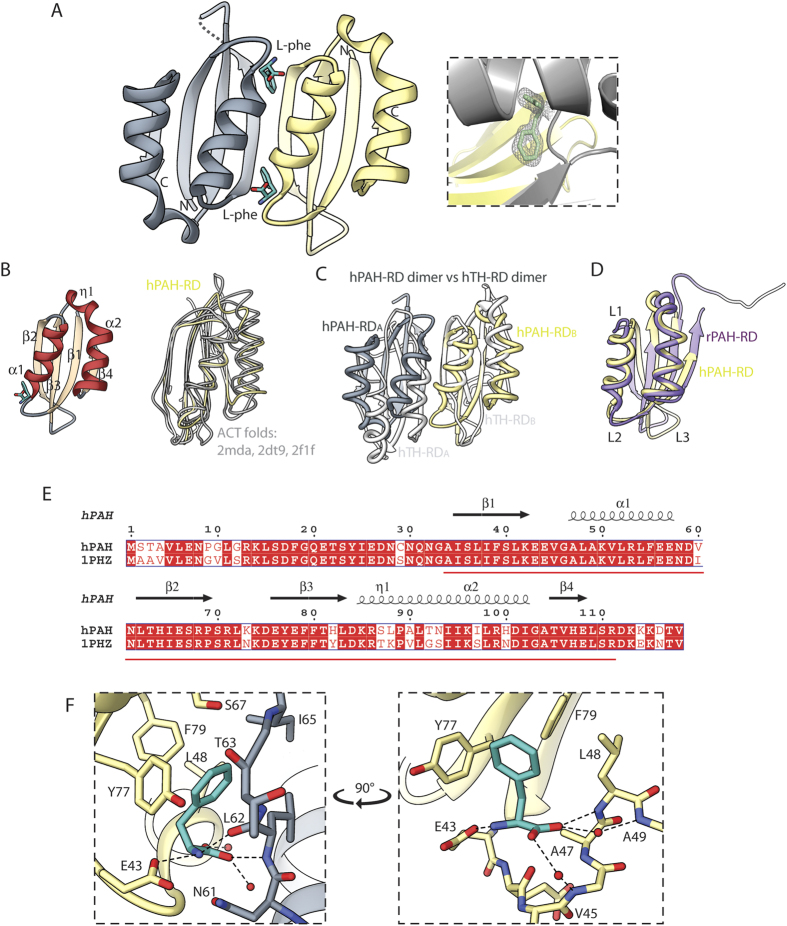
Figure 2. Crystal structure of hPAH-RD.
(A) Ribbon representation of hPAH-RD dimeric structure, coloured grey and yellow for the two subunits. Phe is shown in sticks. (Inset) 2Fo-Fc electron density map showing the bound Phe ligand. (B) Left: Topology of the ACT fold in hPAH-RD. Right: Structural superposition of hPAH-RD (yellow) with other ACT folds (grey) including PDB codes 2 mda, 2dt9 and 2f1f. (C) Structural superposition of hPAH-RD dimer (grey and yellow subunits) with hTH-RD dimer (white subunits). The two subunits of a dimer are annotated as A and B. (D) Structural superposition of hPAH-RD (yellow) with the rat counterpart (rPAH) extracted from its RD + CD structure (purple). (E) Alignment of the RD sequences between hPAH (this study) and rat PAH (PDB code 1 phz). Secondary structures from our hPAH-RD data are shown. Red line denotes the region covered in the hPAH-RD structure. (F) Phe binding site at the dimer interface of hPAH-RD19–118. The two subunits and Phe are colored as in A.