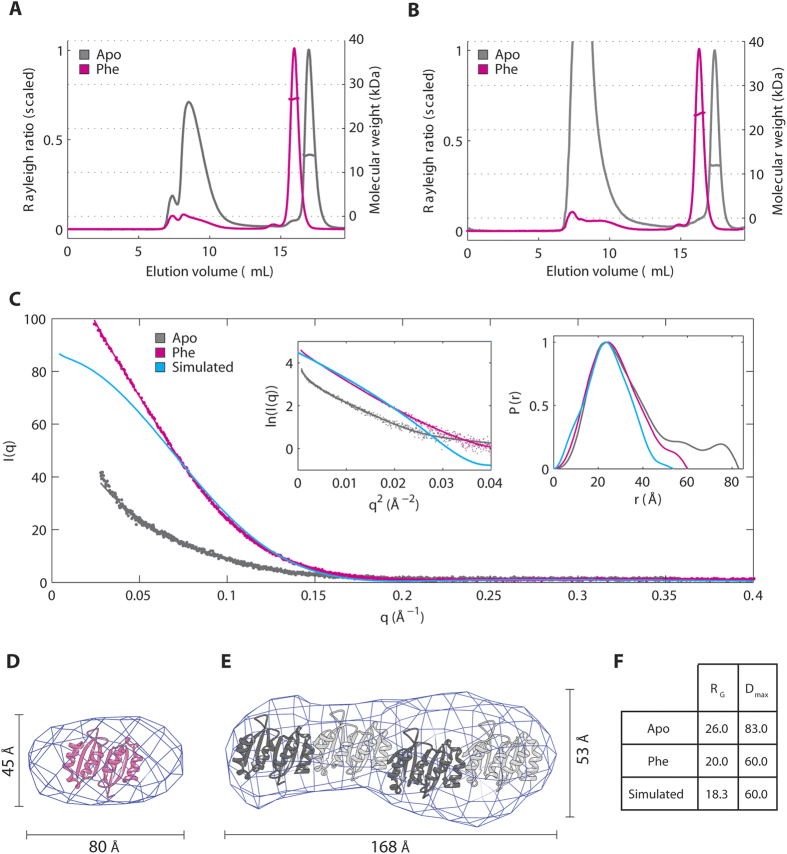
Figure 3. Phenylalanine stabilizes the dimeric conformation of PAH-RD.
(A) SEC-MALS of unliganded (grey line) and Phe-bound (pink line) of hPAH-RD1–118. (B) SEC-MALS of unliganded (grey line) and Phe-bound (pink line) of hPAH-RD19–118. (C) SAXS profiles for hPAH-RD19–118 are plotted for the unliganded (grey line), Phe-bound (pink line) and crystal-structure-simulated (blue line, calculated using CRYSOL) data. Guinier plots (left) and real space P(r) distributions (right) are shown as inset. (D) Ab initio model of Phe-bound hPAH-RD19–118 derived from experimental SAXS data (using a Dmax estimate of 60 Å), superimposed with the crystallographic dimer of hPAH-RD. (E) Ab initio model of unliganded hPAH-RD19–118 derived from experimental SAXS data (using a Dmax estimate of 83 Å), revealing a rod-like shape as the dominant species, potentially accommodating four hPAH-RD crystallographic dimers as modelled. (F) RG and Dmax values calculated for the unliganded, Phe-bound and crystal-structure-simulated SAXS data of hPAH-RD.