Fig. 4.
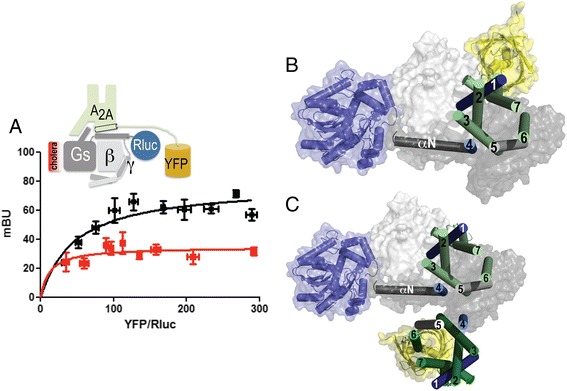
Orientation of a G protein in a receptor homodimer. Bioluminescence resonance energy transfer (BRET) saturation experiments were performed in HEK-293T cells transfected with 2 μg of cDNA corresponding to the α-subunit of Gs fused to Rluc and increasing amounts of A2AR-YFP (0.1–0.5 μg) cDNA. a BRET measurements in cells pretreated for 16 h with medium (black line) or with 100 ng/ml of cholera toxin (red line). Both fluorescence and luminescence of each sample were measured before every experiment to confirm similar donor expressions (approximately 50,000 bioluminescence units) while monitoring the increase in acceptor expression (1000–10,000 fluorescence units). miliBRET unit (mBU) values are the mean ± standard error of the mean of four to five different experiments grouped as a function of the amount of BRET acceptor. A scheme of the placement of donor and acceptor BRET moieties is provided (top). b Molecular model of the A2AR-Gs complex. Rluc (blue) is attached to the N-terminal αN helix of Gs (gray), and YFP (yellow) is attached to the C-terminal domain of A2AR (light green) (see Additional file 9: Figure S9 for details). c Arrangement of A2AR homodimers modeled via the TM4/5 interface as observed in the oligomeric structure of β1-AR [4]. The A2AR protomer bound to αs is shown in light green, whereas the second A2AR-YFP protomer is shown in dark green. The molecular model in panel c (BRET between Rluc in Gs α subunit and YFP in a second A2AR protomer; center-to-center distance between Rluc and YFP of 6.5 nm), in contrast to the model shown in panel B (BRET between Rluc in Gs α subunit and YFP in the G-protein bound A2AR protomer; center-to-center distance between Rluc and YFP of 8.3 nm), would favor the observed high-energy transfer (see panel a) between αs-Rluc and A2AR-YFP
