Sir,
The goal of elimination of leprosy as a public health problem as defined by the World Health Assembly i.e. attaining a level of prevalence of less than one case per 10,000 population, was reached at the global level in 20001. Although India officially declared elimination of leprosy as a public health problem in 2005, 1.27 lakh new leprosy cases were reported in India during 2013-20142. Interactions between Mycobacterium leprae, and the human host and dynamics of its transmission are still not clear. Evidence suggests that the degree of vulnerability of the individual, the extent of exposure and associated environmental factors could potentially influence the transmission. Complete understanding of ecological and environmental components may unfold the gaps in knowledge regarding the mode of transmission of leprosy.
A leprosy vaccine trial from south India3 provided an opportunity for such an ecological exploration. The entire population [covering 148 Panchayats (Rural Administrative Units) comprising 264 contiguous villages from Chingleput district, Tamil Nadu, south India] was screened for leprosy before vaccination. After screening the population, a proportion (5%) was randomly allotted to “blinded” senior officers for quality control. Skin smear examination for detecting acid fast bacilli was done for all suspects and definite cases. A team of independent clinicians visited the field at frequent intervals to monitor the procedures for diagnosis of leprosy. The data collected were validated in many ways with the earlier surveys4. Hence, the quality of data collected was remarkable and comparable to world standard as certified by the independent assessment committee consisting of national and international experts3. The definitions used are explained in detail elsewhere5. Also in the study area, leprosy cases were observed to be geographically clustered. We investigated environmental correlates of leprosy taking into account the spatial dependency using Bayesian model.
Chingleput district in Tamil Nadu State in south India covers an area of 1277 sq km with the minimum and maximum temperatures ranging from 14 to 21°C and 28 to 45°C, respectively. It has an average rainfall of 1200 mm/year and the Normalized Difference Vegetation Index (NDVI) ranging from -0.28 to +0.256. Data from 264 contiguous villages (population size: 300,000) from two taluks (sub-district level administrative unit) in leprosy endemic Chingleput district, were used in the Bayesian model. Total 2098 new leprosy cases (1269 males and 829 females) identified in this population in 2001 have been considered.
We employed a Bayesian model with and without spatial random effect using openBUGS7 software and included demographic data (gender, age, economically higher/poorer strata, and household contacts) as well as environmental and ecological data [rainfall data from Famine Early Warning System (FEWS)8 of South East Asia; Average Day Land Surface Temperature (DLST) and the mean NDVI from Moderate Resolution Imaging Spectroradiometer (MODIS)]9.
For NDVI, rainfall and DLST monthly data for 2001, with a spatial window extent area (12°N - 14°N and 79°E - 81°E) at a resolution of 250 meters that includes the two taluks of south India, were extracted. Annual average for each of the covariate was calculated for each of the data location.
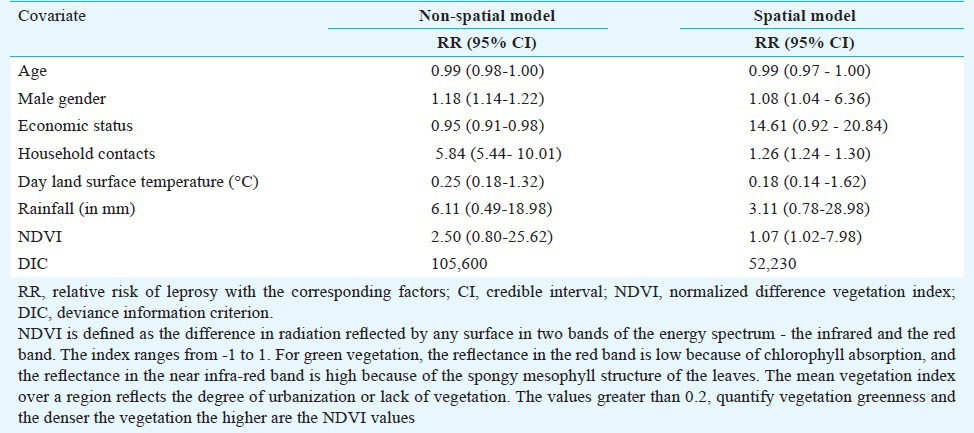
We have assumed that the leprosy status of a respondent at a specified location takes a value of 1 if positive and 0 otherwise, and follows a Bernoulli distribution. Spatial and non-spatial models were compared using the Deviance Information Criterion (DIC)10, being a generalization of the Akaike's Information Criterion in the Bayesian framework, i.e. lower the DIC values better the model. The spatial model with DIC=52,230 outperformed.
It was observed (Table I) that male gender (relative risk, RR=1.08; 95% credible interval, CI=1.04-6.36), household contacts (RR= 1.26; 95% CI=1.24-1.30) and higher NDVI (denser vegetation) (RR=1.07; 95% CI=1.02-7.98) were significantly related to the risk of leprosy. Age, economic status, DLST and rainfall were not related with the risk of leprosy.
Table I.
Median relative risk of leprosy with the factors for two taluks in south India, 2001
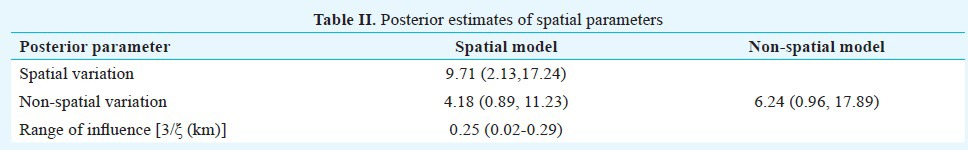
The spatial variation being more compared to non-spatial variation and the range of influence parameter was 250 meters (Table II). The range parameter 3/ξ had a posterior median of 0.25; 95% CI 0.02-0.29. This corresponds to a minimum distance of 250 metres for which the spatial correlation becomes negligible. This indicates a strong spatial correlation in the dataset.
Table II.
Posterior estimates of spatial parameters
Gender and household contacts have been associated with leprosy. Observations made in earlier studies support soil, humidity, vegetation, water, arthropods and armadillos as possible environmental sources/reservoirs of leprosy11,12. Results of an Ethiopian study13 suggest that vertical transmission is not the only mean of acquiring leprosy and viability of M. leprae outside the human body, and the thermal-hydrologic environment also contributes.
Though our study was based on leprosy patients in 2001, they were newly identified and diagnosed patients and not old and prevalent cases. We used corresponding geo-spatial and environmental data of 2001. The significant association observed between NDVI and leprosy cases in Chingelpet district of Tamil Nadu in south India provides additional evidence supporting the role of environmental factors in leprosy transmission. Such factors need to be taken into consideration when planning a control programme. Future field studies may focus more on the risk factors associated with the environmental risk of leprosy.
References
- 1.Pannikar V. Enhanced global strategy for further reducing the disease burden due to leprosy: 2011-2015. Lepr Rev. 2009;80:353–4. [PubMed] [Google Scholar]
- 2.National Leprosy Eradication Programme (NLEP) – Progress Report for the year 2013-14. [accessed on December 20, 2014]. Available from: http://nlep.nic.in/pdf/Progress%20report%2031st%20March%202013-14pdf .
- 3.Gupte MD, Vallishayee RS, Anantharaman DS, Nagaraju B, Sreevatsa, Balasubramanyam S, et al. Comparative leprosy vaccine trial in south India. Indian J Lepr. 1998;70:369–88. [PubMed] [Google Scholar]
- 4.Gupte MD. Leprosy: Epidemiology. In: Valia RG, Valia AR, editors. Text book of atlas of dermatology. 2nd ed. Mumbai: Bhalani Publishing House; 2001. pp. 1543–52. [Google Scholar]
- 5.Joshua V, Gupte MD, Bhagavandas M. A Bayesian approach to study the space time variation of leprosy in an endemic area of Tamil Nadu, South India. Int J Health Geogr. 2008;7:40. doi: 10.1186/1476-072X-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mariappan VEN, Mohanna P. Spatial Urban sprut analysis in Kancheepuram district due to Special Economic Zones (Sez) [accessed on September 10, 2011]. Available from: http://www.academia.edu/1860898/Spatial_Urban_Sprut_Analysis_In_Kancheepuram_District_Due_To_Special_Economic_Zones_Sez .
- 7.Lunn D, Spiegelhalter D, Thomas A, Best N. The BUGS project: Evolution, critique, and future directions. 2009. [accessed on September 10, 2011]. Available from: http://www.openbugs.info/wcgi/Downloads . [DOI] [PubMed]
- 8. [accessed on September 10, 2011]. Available from: http://earlywarning.usgs.gov/fews/southasia/index.php .
- 9. [accessed on September 11, 2011]. Available from: http://modis.gsfc.nasa.gov/data/dataprod/index.php .
- 10.Spiegelhalter DJ, Best NG, Carlin BP, Van derLinde A. Bayesian measures of model complexity and fit. J R Stat Soc B. 2002;64:583–616. [Google Scholar]
- 11.Truman R, Fine PE. Environmental sources of Mycobacterium leprae: issues and evidence. Lepr r. 2010;81:89–95. [PubMed] [Google Scholar]
- 12.Lavania M, Katoch K, Katoch VM, Gupta AK, Chauhan DS, Sharma R, et al. Detection of viable Mycobacterium leprae from environmental soil samples: insights into possible sources for transmission of leprosy. Infect Genet Evol. 2008;8:627–31. doi: 10.1016/j.meegid.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Argaw AT, Shannon EJ, Assefa A, Mikru FS, Miriam BK, Malon JB. A geospatial risk assessment model for leprosy in Ethiopia based on environmental thermal-hydrological regime analysis. Geospat Health. 2006;1:105–13. doi: 10.4081/gh.2006.285. [DOI] [PubMed] [Google Scholar]


