Scheme 4. Enantioselective Alkynylation of Isochroman Acetalsa.
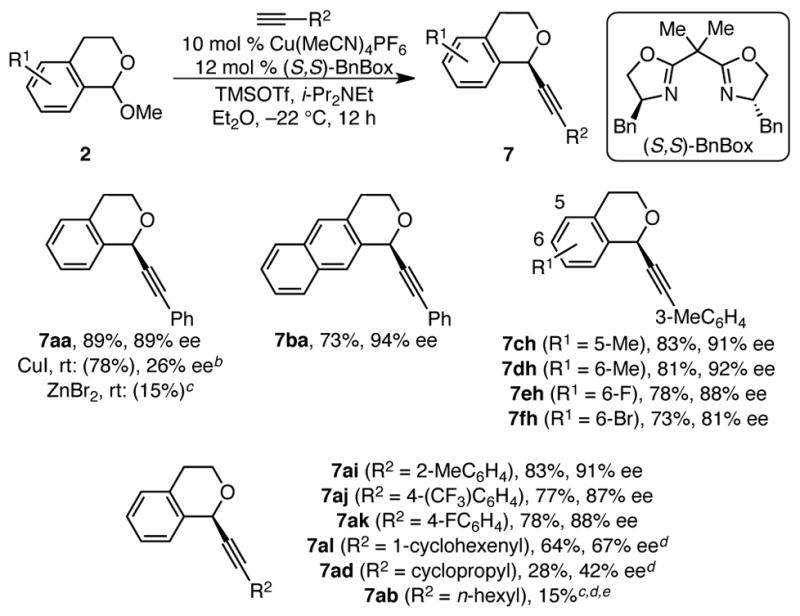
a Conditions: Acetal 2 (0.30 mmol, 1.0 equiv), [Cu(MeCN)4]PF6 (0.030 mmol, 10 mol %), (S,S)-BnBox (0.036 mmol, 12 mol %), alkyne (0.34 mmol, 1.1 equiv), i-Pr2NEt (0.396 mmol, 1.3 equiv), TMSOTf (0.365 mmol, 1.2 equiv, Et2O, −22 °C, 12 h, unless otherwise noted. Average isolated yields (±3%) and ee’s (±2%) from duplicate experiments, unless otherwise noted. Yields in parentheses determined by 1H NMR analysis using 1,3,5-trimethoxybenzene as internal standard. Ee’s determined by HPLC analysis using a chiral stationary phase. b TMSOTf (1.1 equiv), i-Pr2Net (1.2 equiv). c ee not determined. d 20 mol % [Cu], 23 mol % BnBox, PhMe, 0 °C. e 0.1 mmol scale, single experiment.
