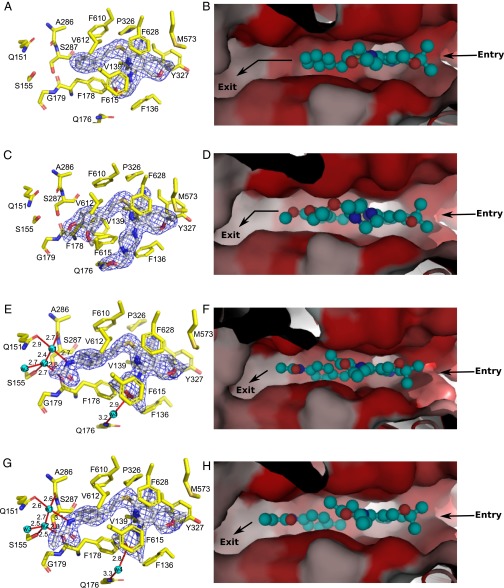
Fig. 3.
Details of the binding of inhibitors with AcrBper. MBX2319 (A and B) and MBX2931 (C and D) mainly interact via hydrophobic stacking interactions with residues comprising the deep binding pocket and the hydrophobic trap. MBX3132 (E and F) and MBX3135 (G and H) are additionally engaged in a water-mediated hydrogen bond network, extending from the acetamide and acrylamide groups, respectively. Hydrogen bonds and water molecules are shown as red lines (with distances in angstroms) and as cyan-colored spheres, respectively. MBX compounds are shown as sticks (carbon, gray; oxygen, red; nitrogen, blue; sulfur, yellow). (Left) AcrB residues involved in inhibitor binding are shown as sticks (carbon, yellow; oxygen, red; nitrogen, blue; sulfur, gold), and the 2Fo-Fc electron density maps (blue-colored mesh) are contoured at 1.0 σ (MBX2319 and MBX2931) and at 1.5 σ (MBX3132 and MBX3135), respectively. (Right) AcrB deep binding pocket surface is colored according to its hydrophobicity (red, hydrophobic; gray, hydrophilic), and the substrate pathway is indicated with arrows. MBX compounds are shown in a ball-and-stick representation (cyan-colored carbon atoms).