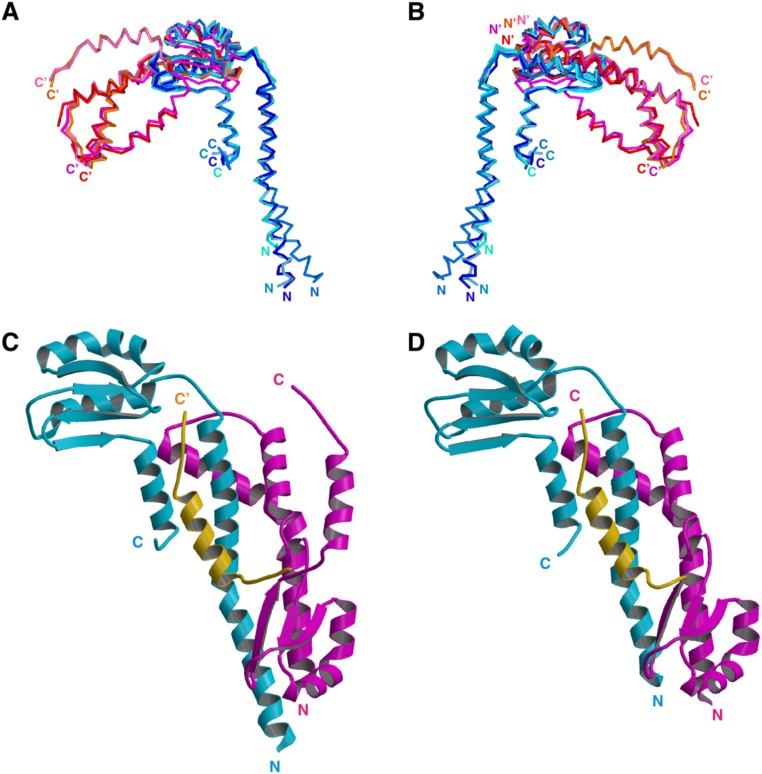
Fig. S3.
Structural variation of FliHC. (A and B) The eight FliHC molecules in the crystallographic asymmetric unit superimposed by fitting the conserved globular domain. (A) Superposition of the Cα backbone trace of FliHC molecules. FliHC-A in trimer-1, -2, -3, and -4 are shown in dark blue, blue, pale blue, and cyan, respectively, and FliHC-B in trimer-1, -2, -3, and -4 are in orange, red, pink, and magenta, respectively. (B) View from the back side of A. The N and C termini are labeled (′ denotes FliHC-B). (C and D) Structural variation in the C-terminal helix of FliHC-B. (C) FliHC2 of trimer-1 shown with the C-terminal helix of FliHC-B of trimer-3 (colored orange). (D) FliHC2 of trimer-2. FliHC-A is colored cyan, FliHC-B is shown in magenta, and the C-terminal helix is highlighted in orange.