Significance
Tyrannosaurs—the iconic group of dinosaurian carnivores that includes Tyrannosaurus rex—dominated latest Cretaceous ecosystems with their colossal sizes and sophisticated senses. A gap in the mid-Cretaceous fossil record between these giant apex predators and their older, smaller relatives makes it difficult to understand how the characteristic body size and ecological habits of T. rex and kin developed. A new species from Uzbekistan fills this gap. This horse-sized animal shows that tyrannosaurs had yet to achieve huge size at this time but had already evolved key brain and sensory features of the gigantic latest Cretaceous species. Tyrannosaurs apparently developed giant body size rapidly, late in the Cretaceous, and their success may have been enabled by their early-evolving keen senses.
Keywords: dinosaur, Tyrannosauroidea, Uzbekistan, phylogenetics, evolution
Abstract
Tyrannosaurids—the familiar group of carnivorous dinosaurs including Tyrannosaurus and Albertosaurus—were the apex predators in continental ecosystems in Asia and North America during the latest Cretaceous (ca. 80–66 million years ago). Their colossal sizes and keen senses are considered key to their evolutionary and ecological success, but little is known about how these features developed as tyrannosaurids evolved from smaller basal tyrannosauroids that first appeared in the fossil record in the Middle Jurassic (ca. 170 million years ago). This is largely because of a frustrating 20+ million-year gap in the mid-Cretaceous fossil record, when tyrannosauroids transitioned from small-bodied hunters to gigantic apex predators but from which no diagnostic specimens are known. We describe the first distinct tyrannosauroid species from this gap, based on a highly derived braincase and a variety of other skeletal elements from the Turonian (ca. 90–92 million years ago) of Uzbekistan. This taxon is phylogenetically intermediate between the oldest basal tyrannosauroids and the latest Cretaceous forms. It had yet to develop the giant size and extensive cranial pneumaticity of T. rex and kin but does possess the highly derived brain and inner ear characteristic of the latest Cretaceous species. Tyrannosauroids apparently developed huge size rapidly during the latest Cretaceous, and their success in the top predator role may have been enabled by their brain and keen senses that first evolved at smaller body size.
Tyrannosaurs were at their heyday during the final ∼20 million years of the Age of Dinosaurs. Iconic taxa like Tyrannosaurus, Tarbosaurus, and Albertosaurus reigned at the top of the food chain in Asia and North America, endowed with colossal size and sophisticated senses that set them apart from other carnivorous dinosaurs (1, 2). These multiton, ecologically dominant latest Cretaceous species (tyrannosaurids) evolved from an ancestral lineage of basal tyrannosauroids, which originated more than 100 million years before T. rex but for most of their history remained second-tier predators, rarely with a mass exceeding that of a horse (2–4). The ascent of tyrannosaurs from these early species to the latest Cretaceous giants was one of the seminal events in dinosaur evolution, establishing the final dinosaur-dominated faunas that flourished before the end-Cretaceous mass extinction (5).
Little is known, however, about how tyrannosaurids developed many of their signature features, such as their gigantic sizes, highly unusual brains, ears attuned to low-frequency sounds, and extensively pneumatized skulls. This is due to a vexing gap in the fossil record that has long frustrated attempts to understand tyrannosaur evolution. There are hundreds of specimens of large-bodied latest Cretaceous tyrannosaurids (1), and now a growing record of smaller and more primitive Middle Jurassic−Early Cretaceous tyrannosauroids (2, 6–8). However, there are no diagnostic fossils from the intervening 20+ million years of the mid-Cretaceous, the time when tyrannosauroids transitioned from marginal hunters to apex predators (1, 2).
We here report the first diagnostic tyrannosauroid from the mid-Cretaceous, a new species from the Turonian (ca. 90–92 million years ago) Bissekty Formation of Uzbekistan. This formation has recently emerged as one of the most important records of mid-Cretaceous dinosaurs globally (9–11). Possible tyrannosauroid specimens from the Bissekty Formation were reported more than a half century ago (12), and, more recently, several isolated fossils were assigned to the group (9, 13), but none of these has been particularly complete or diagnostic at the species level, and their phylogenetic relationships have been difficult to assess. We describe a remarkably preserved and highly diagnostic braincase that links together these specimens and reveals the existence of a midsized species phylogenetically intermediate between the oldest, smallest tyrannosauroids and the largest, last-surviving tyrannosaurids, which had already developed many of the apomorphic features of the tyrannosaurid brain and ear but not the cranial sinus system.
Systematic Paleontology
Dinosauria Owen, 1842; Theropoda Marsh, 1881; Coelurosauria Huene, 1914; Tyrannosauroidea Osborn, 1905; Timurlengia euotica gen. et sp. nov.
Holotype
ZIN PH (Paleoherpetological Collection, Zoological Institute, Russian Academy of Sciences, Saint Petersburg, Russia) 1146/16, a well-preserved braincase missing only the paroccipital processes and much of the parabasisphenoid (Figs. 1 and 2 and Figs. S1−S3).
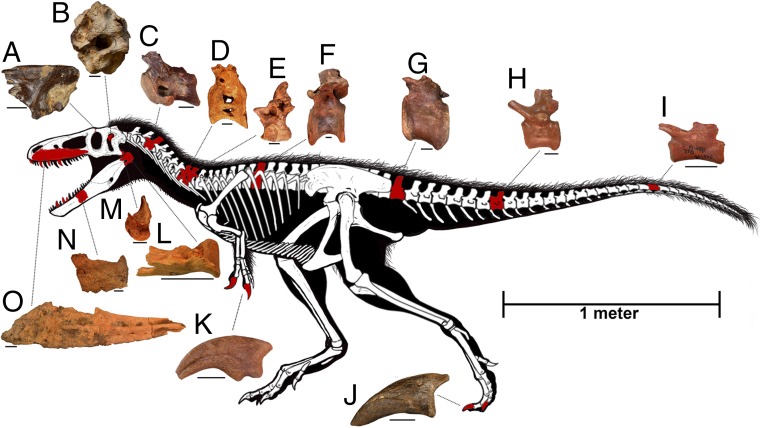
Fig. 1.
Skeletal reconstruction of T. euotica, with known bones colored in red. Individual bones come from different individuals, as they were surface-collected as isolated specimens in the Bissekty Formation of Uzbekistan. The proportions of the skeleton are based on an intermediate body type between Xiongguanlong and Tyrannosaurus but should be considered provisional until associated material is found. Bones are as follows: A, left frontal, ZIN PH 2330/16; B, holotypic braincase, ZIN PH 1146/16; C, cervical vertebra, ZIN PH 671/16; D, cervical vertebra, USNM (National Museum of Natural History) 538131; E, dorsal neural arch, USNM 538132; F, dorsal vertebra, CCMGE (Chernyshev’s Central Museum of Geological Exploration) 432/12457; G, anterior caudal vertebra, ZIN PH 951/16; H, middle caudal vertebra, ZIN PH 120/16; I, distal caudal vertebra, ZIN PH 507/16; J, pedal ungual, USNM 538167; K, manual ungual, ZIN PH 619/16; L, right articular and surangular (reversed), ZIN PH 1239/16; M, left quadrate, ZIN PH 2296/16; N, right dentary, ZIN PH 15/16; and O, right maxilla (reversed), ZIN PH 676/16. (Individual scale bars, 2 cm.) Skeletal drawing courtesy of Todd Marshall.
Fig. 2.
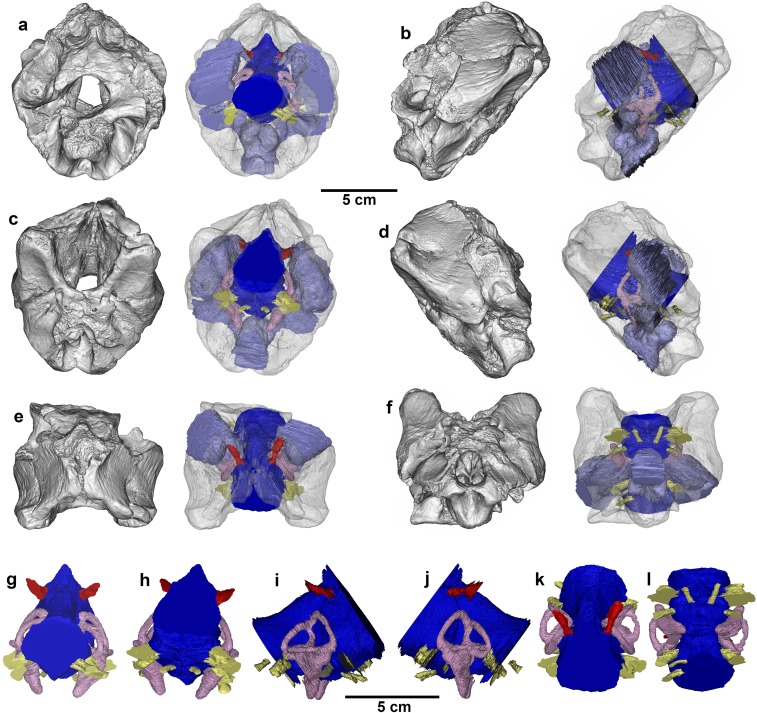
Holotypic braincase of T. euotica (ZIN PH 1146/16). (A) Posterior view. (B) Anteroventral view. (C) Right lateral view. (D) CT reconstruction in posterior view. (E) CT reconstruction of endocast, ear, nerves, and vessels in posterior view. (F) CT reconstruction of endocast in right lateral view. (G) CT reconstruction in right lateral view. Abbreviations are as follows: ac, antotic crest; asc, anterior semicircular canal; bsr, basisphenoid recess; bt, basal tubera; cc, common crus; cd, cochlear duct; ct, crista tuberalis; fl, flocculus; fm, foramen magnum; lsc, lateral semicircular canal; mdp, median dural peak; oc, occipital condyle; or, otic recess; otc, otosphenoidal crest; pcp, paracondylar pocket; pmcv, posterior middle cerebral vein; psc, posterior semicircular canal; ptr, posterior tympanic recess; sor, supraoccipital ridge; sovp, supraoccipital ventral process; ts, transverse sinus; vf, vagal foramen. Roman numerals designate cranial nerves.
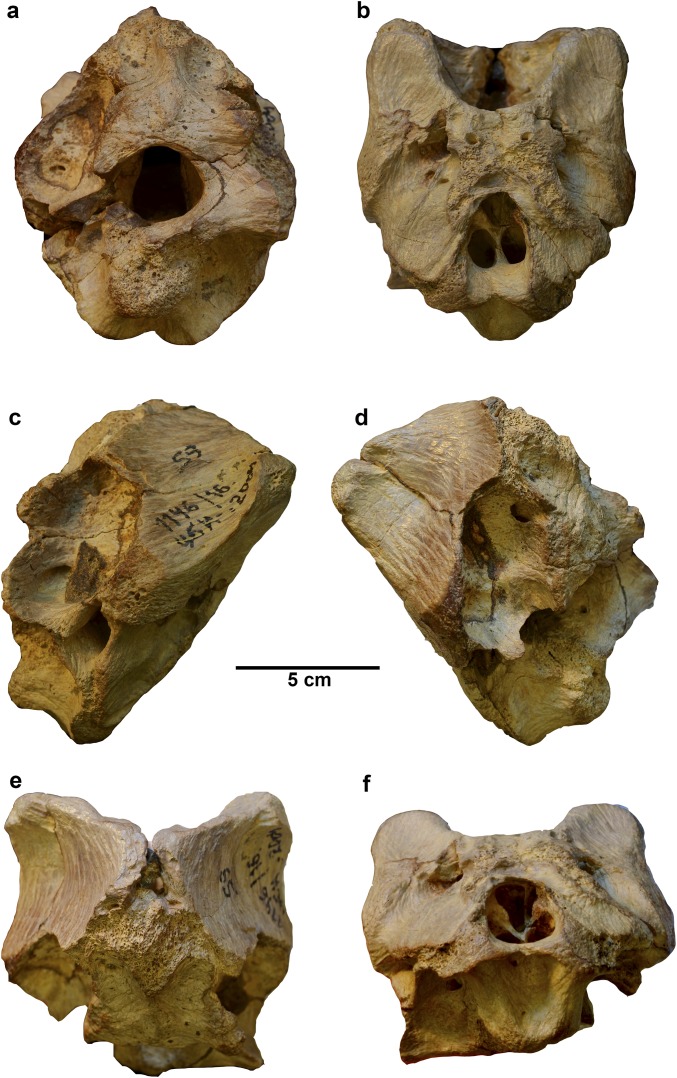
Fig. S1.
Holotypic braincase of T. euotica (ZIN PH 1146/16). (A) Posterior view. (B) Anteroventral view. (C) Right lateral view. (D) Left lateral view. (E) Dorsal view. (F) Ventral view.
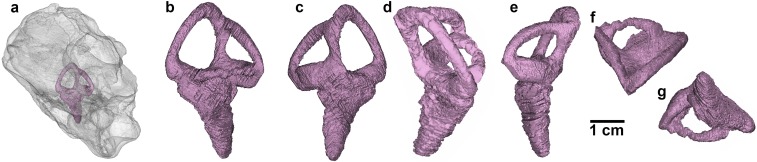
Fig. S3.
Left inner ear of the holotypic braincase of T. euotica (ZIN PH 1146/16). (A) Left lateral view of skull with ear in situ. (B) Lateral view. (C) Medial view. (D) Anterior view. (E) Posterior view. (F) Dorsal view. (G) Ventral view.
Etymology
Timurlengia, in reference to the fourteenth-century Central Asian ruler Timurleng (English: Tamerlane), and euotica, meaning “well-eared” in reference to the large inner ear of the holotype.
Referred Specimens
ZIN PH 854/16 is the right side of a partial braincase that shares unique apomorphies with, and is otherwise identical to, the holotype (Fig. S4). We also refer to T. euotica a series of isolated cranial and postcranial bones described by Averianov and Sues (9) and identified as belonging to indeterminate tyrannosauroids (Fig. 1). These bones possess tyrannosauroid characters, are from the same horizon as the holotype of T. euotica, and belong to individuals of approximately the same body size. Furthermore, separate phylogenetic analyses place the braincase and the series of additional bones in the same position as an “intermediate”-grade tyrannosauroid (see Systematics). We therefore consider it most parsimonious that all of these specimens belong to the same taxon. If later discoveries show this to be incorrect, then the name bearer of T. euotica is the highly diagnostic braincase.
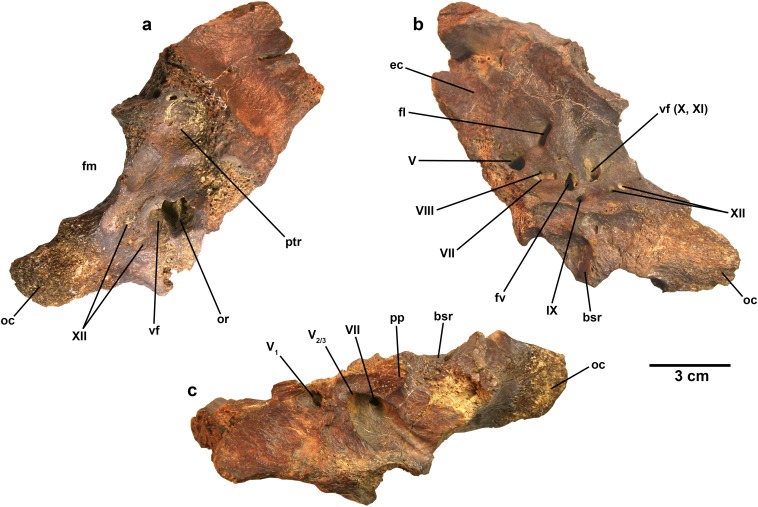
Fig. S4.
Referred partial braincase (fragment of the right side of the braincase) of T. euotica (ZIN PH 854/16). (A) Right lateral view. (B) Medial view. (C) Ventral view. Abbreviations are as follows: bsr, basisphenoid recess; ec, endocranial cavity; fl, flocculus; fm, foramen magnum; fv, fenestra vestibuli; oc, occipital condyle; or, otic recess; pp, preotic pendant; ptr, posterior tympanic recess; vf, vagal foramen. Roman numerals designate cranial nerves.
Horizon and Locality
Dzharakuduk, central Kyzylkum Desert, Navoi Viloyat, Uzbekistan. Bissekty Formation, Upper Cretaceous, Middle-Upper Turonian (ca. 90–92 million years ago).
Diagnosis
The following diagnosis focuses on the holotypic braincase only (Fig. 2 and Figs. S1 and S2). T. euotica is a tyrannosauroid theropod with the following autapomorphies among tyrannosauroids (those unique among all theropods are denoted by an asterisk): a diamond-shaped ventral projection of the supraoccipital that is excluded from the dorsal rim of the foramen magnum*; extremely short basal tubera on the basioccipital, which are approximately one third the depth of the occipital condyle; a deep funnel-like otic recess (combined fenestrae ovalis and vestibuli) that widely opens onto the lateral surface of the braincase and extends far medially into the ear region; and large inner ear with robust semicircular canals*.
Fig. S2.
CT reconstructions of the holotypic braincase of T. euotica (ZIN PH 1146/16). (A) Posterior view. (B) Right lateral view. (C) Anterior view. (D) Left lateral view. (E) Dorsal view. (F) Ventral view. Endocast in (G) anterior view, (H) posterior view, (I) right lateral view, (J) left lateral view, (K) dorsal view, and (L) ventral view.
Body Size
The holotypic braincase of T. euotica is nearly identical in size to that of the holotype of Xiongguanlong baimoensis (based on nearly equal widths of the occipital condyle and supraoccipital above the foramen magnum, and depth of the proximal end of the paroccipital process). Along with the phylogenetic proximity of the two taxa, this suggests that the holotypic individual of T. euotica was roughly the same body size as Xiongguanlong, whose mass has been estimated at 170–270 kg (14, 15). Although most sutures on the holotype of T. euotica are fused, part of the broken parabasisphenoid was not fused to the remainder of the braincase, perhaps suggesting that the individual was not yet osteologically mature and adults of the species may have been somewhat larger. However, almost all other tyrannosauroid specimens from the Bissekty Formation are of the same size, or smaller, than corresponding bones in the holotype of X. baimoensis (9). The one possible exception is a fragment of the posterior end of the lower jaw that was described as belonging to a fully grown adult, which cannot be directly compared with Xiongguanlong (the lower jaw of which is unknown) but is of the approximate size of the corresponding bones in subadult tyrannosaurids (16). Taken together, the known record of Bissekty tyrannosauroids indicates a single taxon that, at adult size, was far smaller than the derived tyrannosaurids of the latest Cretaceous.
Description and Comparisons
The supraoccipital bears a pronounced midline ridge with a deep fossa on each side, as in basal tyrannosauroids (14) and close outgroups (17, 18) but unlike the flatter supraoccipital of derived tyrannosaurids that expands dorsally into tab-like processes (19, 20). Ventrally, the supraoccipital terminates in a large diamond-shaped process that does not extend to the dorsal rim of the foramen magnum. A similar process is present in Xiongguanlong and tyrannosaurids, but it contributes to the foramen rim (14, 20–22). The process is absent and the supraoccipital is broadly separated from the rim in Guanlong and Dilong (20), whereas noncoelurosaurian outgroups have a tiny squared-off projection that enters into the rim (17, 18), and more derived coelurosaurs such as dromaeosaurids have a supraoccipital that broadly contributes to the foramen magnum (23). The supraoccipital is solid internally and lacks the enlarged extensions of the posterior tympanic recesses that fill the bone in tyrannosaurids, including juveniles (20, 24–26).
The bases of the paroccipital processes are entirely hollowed out by enormous posterior tympanic recesses. Only coelurosaurs possess such extensive recesses (20, 27), as outgroups have either a tiny sinus penetrating the anterior edge of the paroccipital process (28) or lack a recess in this region altogether (29, 30). There is a deep fossa on the posterior surface of each paroccipital process, lateral to the foramen magnum, as in basal tyrannosauroids such as Guanlong and Dilong but not in tyrannosaurids (2, 20). A paracondylar pocket housing three foramina—two for cranial nerve XII and a multipurpose vagal foramen for cranial nerves X and XI and associated features—invades the otoccipital and basioccipital dorsolateral to the occipital condyle. The pocket is shallow as in basal tyrannosauroids, not deeply concave as in tyrannosaurids (2, 20). There is no such pocket in dromaeosaurids and other derived coelurosaurs, in which the nerve foramina are flush with the external surface of the otoccipital (23, 31–33).
The basal tubera are autapomorphically small. This is an extreme version of the basal tyrannosauroid condition, in which the basal tubera are shallower than the occipital condyle, in contrast to the longer tubera of tyrannosaurids (2, 20) and derived maniraptorans (23). The basioccipital is not invaded by a subcondylar recess: the interior of this bone is solid, and there are no large pneumatic foramina ventrolateral to the occipital condyle. This recess is characteristic of derived tyrannosaurids (20, 26) and other coelurosaurs (27), but is absent in Xiongguanlong (14) and other basal tyrannosauroids (2, 20). As in most theropods, there is a deep basisphenoid recess on the ventral surface. Its internal structure is complex: posteriorly, it is divided into two large ovoid chambers, and, anteriorly, it is traversed by many small bony struts. Anterior to the recess, much of the parabasisphenoid is missing, as indicated by an open crescentic suture.
A thick crista tuberalis (= metotic strut) separates the posterior and lateral surfaces of the braincase. In posterior view, the width across the opposing cristae is greater than half the depth of the braincase as in Guanlong, Dilong, and Xiongguanlong, but unlike the narrower cristae in tyrannosaurids (20), other coelurosaurs (23, 31–33), and outgroups (17, 18). The otic recess (= columellar canal) is a deep funnel whose wide external opening trends medially into the braincase interior, where it is divided into a pneumatic opening supplying the posterior tympanic recess and the fenestrae vestibuli and pseudorotunda. A deep otic recess with an internal fenestra vestibuli is characteristic of tyrannosauroids (20, 26), and Timurlengia is most similar to Dilong in having a large external opening of the recess, whereas, in tyrannosaurids, the recess is partially concealed by the superficial lamina of the prootic (20).
Extending anterodorsal from the otic recess is a pronounced otosphenoidal–antotic crest on the lateral braincase surface, as in derived tyrannosaurids (2). Anteroventral to this crest are three prominent foramina: one opening for cranial nerve VII and separate openings for the ophthalmic and maxillary–mandibular branches of cranial nerve V. There are no pneumatic foramina in this region. This structure is common among theropods but differs from the highly derived condition of tyrannosaurids, in which a deep prootic fossa houses the nerve openings and a pneumatic opening leading into the anterior tympanic recess (20). The trigeminal nerve (V) emerges from the endocast as a single stalk and bifurcates within the prootic, as is typical for theropods but differs from tyrannosaurids in which the two branches arise from the endocast separately (20, 26). A small portion of the prootic is filled by what may be part of the anterior tympanic recess, but this sinus was probably mostly within the missing parabasisphenoid.
The brain endocast is strikingly similar to those of tyrannosaurids, particularly Alioramus (20, 26, 34, 35). There is only a slight midbrain flexure, resulting in a fairly shallow endocast that would have been tubular if complete. This is a synapomorphy of tyrannosauroids (26), differing from the deeper and more flexed (S-shaped) endocasts of noncoelurosaurian (26, 29) and coelurosaurian (31, 32) outgroups. There is a pronounced midbrain peak (probably related to the transverse and longitudinal venous sinus) anterior to the posterior middle cerebral vein, which is present in tyrannosauroids but absent in most outgroups (20, 26). The flocculus is indistinct and barely crosses the plane of the anterior semicircular canal, as in tyrannosauroids and noncoelurosaurian theropods but contrasting with the enlarged bulbous shape in more derived coelurosaurs (20, 26).
The inner ear endocast is triangular in lateral view with an expanded anterior semicircular canal, as is typical for theropods (26, 29). The semicircular canals and cochlear duct are extremely robust compared with all other theropods with comparative CT data, including both juvenile and adult tyrannosaurids (20, 26, 29). This is an autapomorphy of Timurlengia whose functional implications are unclear, but it could potentially be related to increased agility (36). The cochlear duct is elongated, such that it extends far ventral to the brain endocast and is approximately as long dorsoventrally as the depth of the semicircular canals. A long duct is a synapomorphy of tyrannosauroids (20, 26), different from the much shorter ducts of most other theropods, and would have increased sensitivity to lower-frequency sounds (37, 38) and may possibly have been related to complex vocalizations and sociality (38).
Systematics
To test the phylogenetic relationships of T. euotica broadly among theropods, we added it to the most recent version of the Theropod Working Group dataset (33), which includes a wide sample of coelurosaurs and outgroups scored for over 850 anatomical characters.
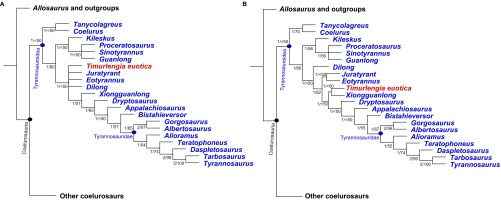
We first ran an analysis in which only the holotypic braincase was used to score Timurlengia, which resulted in 99,999 most parsimonious trees (the memory limit of the program; individual trees: length = 3,364, consistency index = 0.322, retention index = 0.777). The strict consensus places Timurlengia as an intermediate-grade tyrannosauroid, nested between the basal proceratosaurids and the derived large-bodied tyrannosaurids (Fig. S5A). We then undertook a second analysis in which Timurlengia was scored based only on the series of referred specimens described by Averianov and Sues (9). This produced 99,999 most parsimonious trees (length = 3,364, consistency index = 0.322, retention index = 0.777), the strict consensus of which (Fig. S5B) recovered Timurlengia and Xiongguanlong as a sister-taxon pair of intermediate-grade tyrannosauroids. Finally, we ran a third analysis in which Timurlengia was scored based on the holotypic braincase and all additional material described by Averianov and Sues (9). This produced 99,999 most parsimonious trees (length = 3,367, consistency index = 0.322, retention index = 0.777), the strict consensus of which (Fig. 3) places Timurlengia as an intermediate tyrannosauroid, immediately outside the clade of Xiongguanlong and tyrannosaurids.
Fig. S5.
Results of phylogenetic analyses to determine the placement of T. euotica (ZIN PH 1146/16). (A) Strict consensus of 99,999 most parsimonious trees (length = 3,364, consistency index = 0.322, retention index = 0.777) recovered from cladistic analysis in which T. euotica is scored based on the holotypic braincase only. (B) Strict consensus of 99,999 most parsimonious trees (length = 3,364, consistency index = 0.322, retention index = 0.777) recovered from cladistic analysis in which Timurlengia is scored based only on the series of referred specimens described by Averianov and Sues (9). In both cases, numbers next to nodes denote Bremer/jackknife support values.
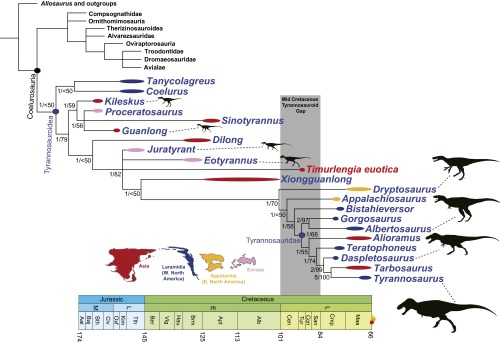
Fig. 3.
Phylogenetic relationships of T. euotica among theropod dinosaurs. Strict consensus of 99,999 most parsimonious trees (length = 3,367, consistency index = 0.322, retention index = 0.777) recovered from cladistic analysis in which T. euotica is scored based on the holotypic braincase and series of referred specimens. Numbers next to nodes are Bremer/jackknife support values, thick lines next to each taxon depict temporal range (which in most cases is age uncertainty and not true range), colors of lines denote geographic areas, and silhouettes are in relative proportion and scaled to total body length (T. rex = 13 m). Geographic silhouettes are from Loewen et al. (3), and taxon silhouettes are courtesy of phylopic.org (Kileskus, T. M. Keesey; Guanlong, S. Hartman; Juratyrant, S. Hartman, T. M. Keesey; Eotyrannus, S. Hartman; Dryptosaurus, T. M. Keesey; Albertosaurus, C. Dylke; Daspletosaurus, S. O’Connor, T. M. Keesey; Tyrannosaurus, S. Hartman).
We interpret these results as supporting two main conclusions. First, the braincase and referred specimens belong to the same taxon, given their nearly identical phylogenetic placement when run separately and the absence of any fossils indicating the presence of more than one tyrannosauroid taxon in the Bissekty Formation. Second, this taxon, T. euotica, is an intermediate-grade tyrannosauroid that is phylogenetically proximal to Xiongguanlong, and the two may or may not form their own distinct subclade.
Discussion
The discovery of the braincase ZIN PH 1146/16, with its unambiguous tyrannosauroid features but lack of synapomorphies of large-bodied tyrannosaurids, helps tie together disparate specimens of small tyrannosauroids found over many years in the Bissekty Formation of Uzbekistan (9, 12, 13). It reveals the presence of a new taxon that fills a major gap in the evolutionary history of tyrannosauroids, which has long frustrated attempts to understand the phylogeny and evolution of these most iconic of dinosaurs. Critically, the new taxon, T. euotica, is the first diagnostic tyrannosauroid from the middle part of the Cretaceous, an ∼20-million-year window spanning the end of the Albian to the early Campanian. Previous reports of tyrannosauroids from this interval have been limited to fragmentary and undiagnostic specimens (12, 13), have uncertain stratigraphic ages and may be from the latest Cretaceous (39), or are specimens whose ages are poorly constrained and whereabouts currently unknown (40).
Timurlengia helps clarify trends in tyrannosauroid body size evolution, particularly the origin of colossal sizes in latest Cretaceous tyrannosaurids, which, at 10+ m long and 5+ tons in mass, were among the largest carnivores to ever live on land. Some basal tyrannosauroids, such as Yutyrannus (41), evolved fairly large size (∼8–9 m long, 1.5 tons in mass) in the Early Cretaceous, but these were phylogenetically basal taxa that developed their large size independently of the latest Cretaceous species (4). There is a general increase in body size across tyrannosauroid phylogeny (4, 14), but a marked change occurred sometime in the Middle Cretaceous gap, when tyrannosauroids ascended into an apex predator role. The last-known species before the gap, about 100–125 million years ago, were about the size of a horse, and the first ones appearing after the gap, about 80 million years ago, are gigantic taxa such as Lythronax and Gorgosaurus (2, 3). The new mid-Cretaceous Uzbek taxon is also approximately horse-sized, far smaller than the end-Cretaceous giants. Although it is currently only a single data point, Timurlengia indicates that tyrannosauroids remained small-to-medium-sized well into the Middle Cretaceous, during a time when late-surviving large allosauroids remained at the top of food chains in Asia and North America (42–44). Tyrannosauroids apparently developed huge size and ecological dominance suddenly, sometime around the start of the Campanian, but the trigger remains unclear. It is tempting to speculate that the Cenomanian−Turonian extinction event ca. 94 million years ago may have played a role, but this may have occurred many millions of years before the allosauroid−tyrannosauroid turnover. Unfortunately, the currently poorly known mid-Cretaceous fossil record makes testing extinction and radiation scenarios difficult.
The new Uzbek taxon is an intermediate tyrannosauroid, within a grade of species between the oldest and most primitive proceratosaurids (Guanlong, Proceratosaurus, and kin: refs. 6–8) and the derived, large-bodied tyrannosaurids (Tyrannosaurus, Albertosaurus, and kin: refs. 21–22, 25). Timurlengia shares many similarities with another intermediate taxon, Xiongguanlong from the Aptian−Albian of western China, which is the last-known diagnostic tyrannosauroid before the mid-Cretaceous gap (14). Their braincases are almost identical in size, they possess nearly identical diamond-shaped ventral processes of the supraoccipital (differing only in whether the process makes contact with the dorsal rim of the foramen magnum), and both have short basal tubera without large external pneumatic openings leading into a subcondylar recess. Furthermore, one of the referred specimens of Timurlengia is a gracile and elongate maxilla, remarkably similar in proportions to that of the long-snouted Xiongguanlong. We hypothesize that these two taxa, and, potentially, a long-snouted skull from the Bayan Shireh Formation previously assigned to Alectrosaurus but unavailable for study (40), belong to a grade or clade of longirostrine tyrannosauroids immediately outside of the clade of derived, large-bodied, latest Cretaceous forms. This midsized, long-snouted bauplan may have been the ancestral stock from which the gigantic tyrannosaurids arose.
The holotypic braincase of T. euotica is a keystone specimen for understanding how the highly derived brains, sensory organs, and endocranial sinuses of large-bodied tyrannosaurids evolved. Braincase material is known for only a few other basal tyrannosauroids, but has yet to be CT scanned or studied in detail, making Timurlengia the oldest and most basal tyrannosauroid with a well-documented braincase. Aside from its autapomorphically robust inner ear, the brain of Timurlengia resembles a smaller version of the brain of Gorgosaurus or Tyrannosaurus (25, 26). It reveals that many apomorphic features of the tyrannosaurid brain and ear were already present in midsized, nontyrannosaurid taxa, including a tubular endocast with a slight midbrain flexure, a pronounced midbrain peak, and an elongate cochlear duct. It has been suggested that some of these features may have been instrumental in the evolutionary success of the large-bodied, latest Cretaceous tyrannosaurids, particularly the long cochlear duct that imparts heightened ability to hear lower-frequency sounds than in other theropods, which would have been useful for an apex predator (2, 26). These features, however, developed long before large body size. In this regard, the brains and keen senses of early tyrannosauroids may have predisposed them to become successful apex predators when the opportunity arose. This has parallels to the “head-first” model, in which characteristic features of the oversized and robust tyrannosaurid skull evolved before those of other regions of the skeleton, noted by some authors (41, 45).
On the other hand, the pneumatic sinuses of Timurlengia are nowhere near as elaborate as in the largest tyrannosaurids. The baseline sinus system of coelurosaurs is present, but Timurlengia lacks the supraoccipital and subcondylar recesses so characteristic of tyrannosaurids, and also seemingly possesses a less extensive anterior tympanic recess in the prootic. It may be that these recesses developed in concert with large body size, either to lighten the skull or, in the case of the tympanic sinuses, to help tyrannosaurids maintain the ability to hear lower-frequency sounds at larger size (26).
Conclusions
Timurlengia is a long-awaited diagnostic tyrannosauroid from the middle part of the Cretaceous. It indicates that these predators were still far from giants during this time, but had already evolved signature brain and sensory features that may have been tied to the extraordinary success of the last-surviving, latest Cretaceous species like Tyrannosaurus. However, Timurlengia remains a single data point from a still murky interval in dinosaur history, and future discoveries from this gap will undoubtedly lead to a better understanding of how tyrannosauroids rose from marginal creatures into some of the largest terrestrial predators in Earth history.
Materials and Methods
To visualize and study the internal structures of the braincase, ZIN PH 1146/16 was subjected to an X-ray computed microtomography scan at the School of GeoSciences, University of Edinburgh (µCT instrument constructed in-house). Data were acquired using a Feinfocus transmission X-ray source at a peak energy of 120 kV and a Perkin-Elmer XRD0822 flat panel X-ray detector using 3,000 exposures of 2 s each and an aluminum energy filter. Octopus v8.7 was used for tomographic reconstruction. The final slice datasets were imported into Mimics v.17 for segmentation and digital reconstruction.
To assess the phylogenetic relationships of T. euotica, we added it to the data matrix of Brusatte et al. (33), which is the latest iteration of a 20+ y research program [Theropod Working Group (TWiG)] that has been building progressively larger datasets of theropod phylogeny based on direct examination of specimens. The dataset includes 153 taxa scored for 853 discrete morphological characters, representing a broad spread of theropods and their anatomical features. Three versions of the analysis were run in which T. euotica was scored based on (i) the holotypic braincase only, (ii) the series of specimens described by Averianov and Sues (9) only, or (iii) both the braincase and the series of referred specimens. In each case, the matrix was run in TNT v1.1 (46) under maximum parsimony. We first used a “new technology search” in which the shortest tree was found in 10 replicates, and then those trees were subjected to a “traditional” round of tree bisection and reconnection branch swapping. For further details, see SI Text.
SI Text
SI Materials and Methods
T. euotica was added to the phylogenetic dataset of Brusatte et al. (33), the latest iteration of the Theropod Working Group dataset, which includes 853 characters scored for 153 taxa. A few other changes to the dataset were made: (i) Incisivosaurus, Guanlong, Dilong, Alioramus, Daspletosaurus, and Tyrannosaurus were scored as state 1 for character 7. (ii) Sinraptor was scored as state 1 for character 18; and (iii) Xiongguanlong was scored as state 1 for character 609. The complete dataset is provided in Dataset S1.
Three separate phylogenetic analyses were run, differing only in how T. euotica was scored: (i) based on the holotypic braincase only, (ii) based on the referred specimens described by Averianov and Sues (9) only, and (iii) based on the holotypic braincase and the referred specimens.
In each case, the dataset was analyzed with equally weighted parsimony in the phylogenetic program TNT v. 1.1 (46). Following previous TWiG protocol, the outgroup Allosaurus was used to root the tree. Because of the large size of the dataset, a heuristic search strategy was necessary. First, the data matrix was analyzed under the “New Technology” search options, using sectorial search, ratchet, tree drift, and tree fuse options with default parameters. The minimum length tree (=most parsimonious tree, MPT) was found in 10 replicates; a procedure samples as many tree islands as possible. The generated trees were then analyzed under traditional tree bisection and reconnection branch swapping, which aims to more fully explore each tree island. Zero-length branches were collapsed following Rule 1 of Coddington and Scharff (47).
Strict consensus topologies were constructed for each analysis after exclusion of five wildcard taxa: Kinnareemimus, Epidendrosaurus, Pyroraptor, Hesperonychus, and Limenavis (see ref. 33). The degree of support for individual clades was assessed in two ways: Bremer support values (= decay indices, the number of extra steps required for the clade to fall apart in the strict consensus of less optimal topologies) and jackknife percentages (the percent of trees which includes the clade in question recovered in a jackknife analysis, with 36% character removal probability and 500 replicates).
We note that, because of the large size of our dataset and the capabilities of the software, the number of most parsimonious trees that we find hits the memory limit in TNT v. 1.1. This has minimal effect on our results, however, as the large number of MPTs is primarily due to the many (often incomplete) dromaeosaurids, troodontids, and basal avialans included in the dataset. When only the tyrannosauroids and outgroups are analyzed, a small number of MPTs are found (e.g., six for the dataset in which the braincase and other bones are analyzed together), which are consistent with the strict consensus of the 99,999 MPTs from the full analysis (i.e., the Uzbek taxon is found to be an intermediate-grade tyrannosauroid).
Supplementary Material
Acknowledgments
We thank many colleagues for discussion about tyrannosauroids: R. Benson, T. Carr, P. Currie, T. Holtz, J. Horner, M. Loewen, J. Lü, P. Makovicky, M. Norell, K. Padian, P. Sereno, T. Williamson, and X. Xu. T. Marshall skillfully prepared the drawing used in Fig. 1. S.L.B. is supported by Marie Curie Career Integration Grant EC630652 and the University of Edinburgh, and his trip to St. Petersburg was supported by National Science Foundation (NSF) DDIG DEB-1110357. A.A. and H.-D.S. acknowledge support for fieldwork from NSF EAR-9804771 and EAR-0207004 and Grants 5901-97 and 6281-98 from the National Geographic Society. A.A. was supported by the Russian Scientific Fund Project 14-14-00015.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1600140113/-/DCSupplemental.
References
- 1.Holtz TR., Jr . Tyrannosauroidea. In: Weishampel DB, Dodson P, Osmólska H, editors. The Dinosauria. 2nd Ed. Univ Calif Press; Berkeley, CA: 2004. pp. 111–136. [Google Scholar]
- 2.Brusatte SL, et al. Tyrannosaur paleobiology: New research on ancient exemplar organisms. Science. 2010;329(5998):1481–1485. doi: 10.1126/science.1193304. [DOI] [PubMed] [Google Scholar]
- 3.Loewen MA, Irmis RB, Sertich JJW, Currie PJ, Sampson SD. Tyrant dinosaur evolution tracks the rise and fall of Late Cretaceous oceans. PLoS One. 2013;8(11):e79420. doi: 10.1371/journal.pone.0079420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brusatte SL, Carr TD. The phylogeny and evolutionary history of tyrannosauroid dinosaurs. Sci Rep. 2016;6:20252. doi: 10.1038/srep20252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brusatte SL, et al. The extinction of the dinosaurs. Biol Rev Camb Philos Soc. 2015;90(2):628–642. doi: 10.1111/brv.12128. [DOI] [PubMed] [Google Scholar]
- 6.Xu X, et al. A basal tyrannosauroid dinosaur from the Late Jurassic of China. Nature. 2006;439(7077):715–718. doi: 10.1038/nature04511. [DOI] [PubMed] [Google Scholar]
- 7.Rauhut OWM, Milner AC, Moore-Fay S. Cranial osteology and phylogenetic position of the theropod dinosaur Proceratosaurus bradleyi (Woodward, 1910) from the Middle Jurassic of England. Zool J Linn Soc. 2010;158(1):155–195. [Google Scholar]
- 8.Averianov AO, Krasnolutskii SA, Ivantsov SV. A new basal coelurosaur (Dinosauria: Theropoda) from the Middle Jurassic of Siberia. Proc Zool Inst RAN. 2010;314(1):42–57. [Google Scholar]
- 9.Averianov A, Sues H-D. Skeletal remains of Tyrannosauroidea (Dinosauria: Theropoda) from the Bissekty Formation (Upper Cretaceous: Turonian) of Uzbekistan. Cretac Res. 2012;34:284–297. [Google Scholar]
- 10.Sues H-D, Averianov A, Ridgely RC, Witmer LM. Titanosauria (Dinosauria, Sauropoda) from the Upper Cretaceous (Turonian) Bissekty Formation of Uzbekistan. J Vertebr Paleontol. 2015;35(1):e889145. [Google Scholar]
- 11.Averianov A, Sues H-D. Troodontidae (Dinosauria: Theropoda) from the Upper Cretaceous of Uzbekistan. Cretac Res. 2016;59:98–110. [Google Scholar]
- 12.Efremov IA. [Dinosaur horizon of Middle Asia and some questions of stratigraphy] Izv Akad Nauk SSSR Ser Geol. 1944;3:40–58. Russian. [Google Scholar]
- 13.Nessov LA. [Dinosaurs of Northern Eurasia: New Data about Assemblages, Ecology and Paleobiogeography] Izdatelstvo Sankt-Peterburgskogo Univ; Saint Petersburg, Russia: 1995. Russian. [Google Scholar]
- 14.Li D, Norell MA, Gao KQ, Smith ND, Makovicky PJ. A longirostrine tyrannosauroid from the Early Cretaceous of China. Proc Biol Sci. 2010;277(1679):183–190. doi: 10.1098/rspb.2009.0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benson RBJ, et al. Rates of dinosaur body mass evolution indicate 170 million years of sustained ecological innovation on the avian stem lineage. PLoS Biol. 2014;12(5):e1001853. doi: 10.1371/journal.pbio.1001853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brusatte SL, Carr TD, Norell MA. The osteology of Alioramus, a gracile and long-snouted tyrannosaurid (Dinosauria: Theropoda) from the Late Cretaceous of Mongolia. Bull Am Mus Nat Hist. 2012;366:1–197. [Google Scholar]
- 17.Madsen J. Allosaurus fragilis: A revised osteology. Utah Geol Surv Bull. 1976;109:1–163. [Google Scholar]
- 18.Currie PJ, Zhao X-J. A new large theropod (Dinosauria, Theropoda) from the Jurassic of Xinjiang, People’s Republic of China. Can J Earth Sci. 1993;30:2037–2081. [Google Scholar]
- 19.Bakker RT, Williams M, Currie PJ. Nanotyrannus, a new genus of pygmy tyrannosaur, from the latest Cretaceous of Montana. Hunteria. 1988;1(5):1–30. [Google Scholar]
- 20.Bever GS, et al. The braincase anatomy of the Late Cretaceous dinosaur Alioramus (Theropoda: Tyrannosauroidea) Bull Am Mus Nat Hist. 2013;376:1–72. [Google Scholar]
- 21.Currie PJ. Cranial anatomy of tyrannosaurid dinosaurs from the Late Cretaceous of Alberta, Canada. Acta Pal Polonica. 2003;48(2):191–226. [Google Scholar]
- 22.Hurum JH, Sabath K. Giant theropod dinosaurs from Asia and North America: skulls of Tarbosaurus bataar and Tyrannosaurus rex compared. Acta Pal Polonica. 2003;48(2):161–190. [Google Scholar]
- 23.Currie PJ. New information on the anatomy and relationships of Dromaeosaurus albertensis (Dinosauria: Theropoda) J Vertebr Paleontol. 1995;15(3):576–591. [Google Scholar]
- 24.Russell DA. Tyrannosaurs from the Late Cretaceous of western Canada. Nat Mus Nat Sci Pub Palaeontol. 1970;1:1–34. [Google Scholar]
- 25.Brochu CA. Osteology of Tyrannosaurus rex: Insights from a nearly complete skeleton and high-resolution computed tomographic analysis of the skull. Soc Vert Paleontol Mem. 2003;7:1–138. [Google Scholar]
- 26.Witmer LM, Ridgely RC. New insights into the brain, braincase, and ear region of tyrannosaurs (Dinosauria, Theropoda), with implications for sensory organization and behavior. Anat Rec (Hoboken) 2009;292(9):1266–1296. doi: 10.1002/ar.20983. [DOI] [PubMed] [Google Scholar]
- 27.Tahara R, Larsson HCE. Cranial pneumatic anatomy of Ornithomimus edmonticus (Ornithomimidae: Theropoda) J Vertebr Paleontol. 2011;31:127–143. [Google Scholar]
- 28.Paulina Carabajal A, Currie PJ. New information on the braincase of Sinraptor dongi (Theropoda: Allosauroidea): Ethmoidal region, endocranial anatomy, and pneumaticity. Vertebr PalAsiatica. 2012;50(2):85–101. [Google Scholar]
- 29.Sampson SD, Witmer LM. Craniofacial anatomy of Majungasaurus crenatissimus (Theropoda: Abelisauridae) from the Late Cretaceous of Madagascar. Soc Vert Paleontol Mem. 2007;8:32–104. [Google Scholar]
- 30.Brusatte SL, Chure DJ, Benson RBJ, Xu X. The osteology of Shaochilong maortuensis, a carcharodontosaurid (Dinosauria: Theropoda) from the Late Cretaceous of Asia. Zootaxa. 2010;2334:1–46. [Google Scholar]
- 31.Norell MA, Makovicky PJ, Bever GS, Balanoff AM, Clark JM, Barsbold R, Rowe T. A review of the Mongolian Cretaceous dinosaur Saurornithoides (Troodontidae: Theropoda) Am Mus Nov. 2009;3654:1–63. [Google Scholar]
- 32.Balanoff AM, Xu X, Kobayashi Y, Matsufune Y, Norell MA. Cranial osteology of the theropod dinosaur Incisivosaurus gauthieri (Theropoda: Oviraptorosauria) Am Mus Nov. 2009;3651:1–35. [Google Scholar]
- 33.Brusatte SL, Lloyd GT, Wang SC, Norell MA. Gradual assembly of avian body plan culminated in rapid rates of evolution across the dinosaur-bird transition. Curr Biol. 2014;24(20):2386–2392. doi: 10.1016/j.cub.2014.08.034. [DOI] [PubMed] [Google Scholar]
- 34.Bever GS, Brusatte SL, Balanoff AM, Norell MA. Variation, variability, and the origin of the avian endocranium: Insights from the anatomy of Alioramus altai (Theropoda: Tyrannosauroidea) PLoS One. 2011;6(8):e23393. doi: 10.1371/journal.pone.0023393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brochu CA. A digitally-rendered endocast for Tyrannosaurus rex. J Vertebr Paleontol. 2000;20(1):1–6. [Google Scholar]
- 36.Georgi JA, Sipla JS, Forster CA. Turning semicircular canal function on its head: Dinosaurs and a novel vestibular analysis. PLoS One. 2013;8(3):e58517. doi: 10.1371/journal.pone.0058517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manley GA. Peripheral Hearing Mechanisms in Reptiles and Birds. Springer; Berlin: 1990. [Google Scholar]
- 38.Walsh SA, Barrett PM, Milner AC, Manley G, Witmer LM. Inner ear anatomy is a proxy for deducing auditory capability and behaviour in reptiles and birds. Proc R Soc Lon B. 2009;276(1660):1355–1360. doi: 10.1098/rspb.2008.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gilmore CW. On the dinosaurian fauna of the Iren Dabasu Formation. Bull Am Nat Hist. 1933;67:23–78. [Google Scholar]
- 40.Perle A. [On the first discovery of alectrosaur (Tyrannosauridae, Theropoda) from the Late Cretaceous of Mongolia] Problemy Mongol'skoi Geologii. 1977;3:104–113. [Google Scholar]
- 41.Xu X, et al. A gigantic feathered dinosaur from the lower Cretaceous of China. Nature. 2012;484(7392):92–95. doi: 10.1038/nature10906. [DOI] [PubMed] [Google Scholar]
- 42.Benson RBJ, Xu X. The anatomy and systematic position of the theropod dinosaur Chilantaisaurus tashuikouensis Hu, 1964 from the Early Cretaceous of Alanshan, People’s Republic of China. Geol Mag. 2008;145(6):778–789. [Google Scholar]
- 43.Brusatte SL, et al. The first definitive carcharodontosaurid (Dinosauria: Theropoda) from Asia and the delayed ascent of tyrannosaurids. Naturwissenschaften. 2009;96(9):1051–1058. doi: 10.1007/s00114-009-0565-2. [DOI] [PubMed] [Google Scholar]
- 44.Zanno LE, Makovicky PJ. Neovenatorid theropods are apex predators in the Late Cretaceous of North America. Nat Commun. 2013;4:2827. doi: 10.1038/ncomms3827. [DOI] [PubMed] [Google Scholar]
- 45.Sereno PC, et al. Tyrannosaurid skeletal design first evolved at small body size. Science. 2009;326(5951):418–422. doi: 10.1126/science.1177428. [DOI] [PubMed] [Google Scholar]
- 46.Goloboff PA, Farris JA, Nixon KC. TNT, a free program for phylogenetic analysis. Cladistics. 2008;24(5):774–786. [Google Scholar]
- 47.Coddington JA, Scharff N. Problems with zero-length branches. Cladistics. 1994;10(4):415–423. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.