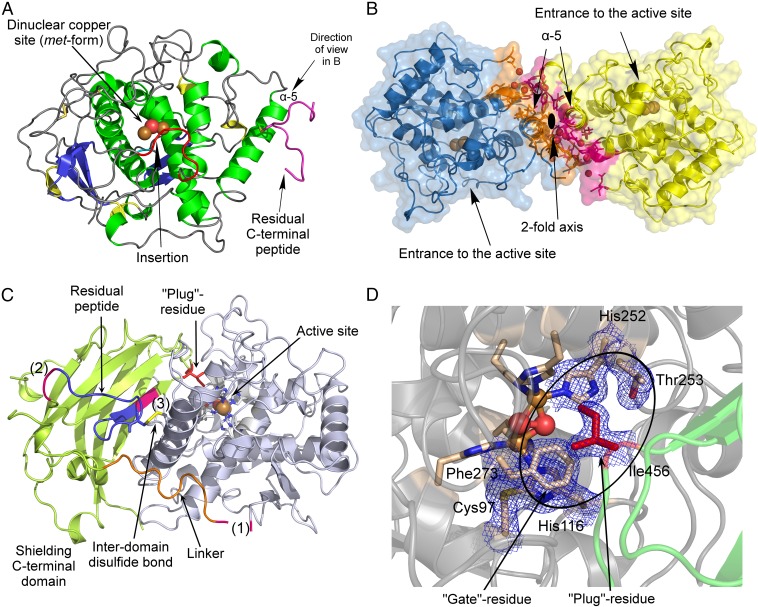
Fig. 1.
Overall structures of latent and mature aurone synthase. (A) Top view of mature aurone synthase. The coloring was performed according to the secondary structure features (α-helices, green; 310-helices, yellow; β-sheets, blue), and the characteristic features of AUS1 are colored red (loop carrying the insertion V237ANG240) and magenta (residual C-terminal peptide). (B) Side view of the dimeric biological assembly of mature AUS1. The interacting residues are colored orange (monomer chain B) and magenta (monomer chain D). (C) Overall structure of latent aurone synthase. The features of the C-terminal domain are as follows: the three proteolytic cleavage sites, colored magenta and labeled as (1), (2), and (3); the linker region (orange) that connects the catalytically active domain (gray) and the shielding C-terminal domain (green); and the residual peptide of the C-terminal domain (blue) and the active site shielding Ile456 (plug residue) (red). (D) The active site is shielded by the C-terminal residue Ile456, which is responsible for the latency of the proenzyme and functions like a plug [2Fo-Fc electron density map (blue mesh) contoured at 1.0 σ].