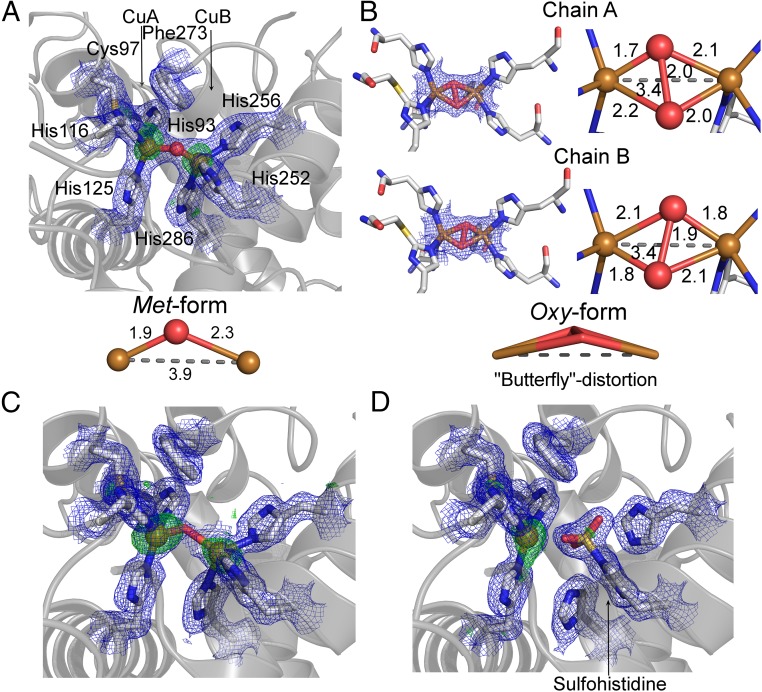
Fig. 3.
Copper binding site of AUS1. The 2Fo-Fc electron density map (blue mesh) is contoured at 1.0 σ, and the anomalous Fourier difference map in A, C, and D (green mesh) is contoured at 3.0 σ. (A) Native met-copper center of recombinantly expressed AUS1. (B) Oxy-copper center of recombinantly expressed AUS1 (μ-η2:η2-peroxo geometry; crystal soaked in H2O2). The oxygen atoms are slightly unsymmetrically bound to the copper atoms, and the μ-η2:η2 peroxo complex displays a butterfly distortion. (C) Copper binding site of active AUS1 (occupancy CuB, ∼0.55). For clarity, the sulfohistidine (occupancy, ∼0.45) is not shown. (D) Inactive AUS1 (occupancy sulfohistidine, 0.9).