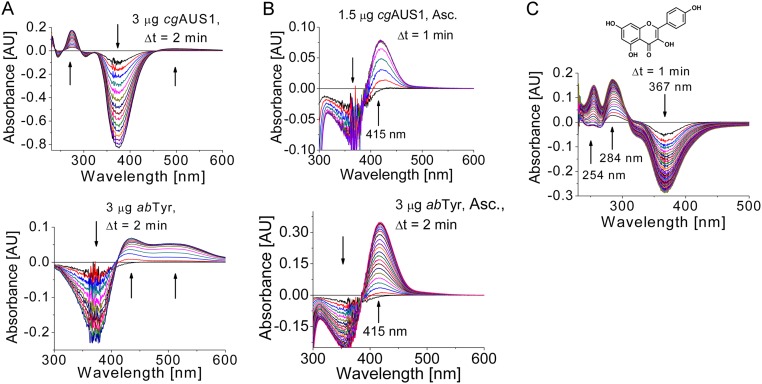
Fig. S5.
Monophenolase activity of AUS1 and its comparison with mushroom tyrosinase (abTYR). The reaction medium (1 mL) contained 50 µM substrate (A and B, isoliquiritigenin; C, kaempferol) in 100 mM sodium phosphate pH 6.5. (A) Comparison of difference spectra of the reaction of isoliquiritigenin catalyzed by AUS1 (Top) and abTYR (Bottom) without a reducing agent. In contrast to the reaction catalyzed by mushroom tyrosinase, only an extremely weak and very broad absorption band at a higher wavelength than expected appears, although the disappearance of substrate is visible. The reason for the occurring different reaction products remains unclear. However, the comparison of the enzymes in the presence of a reducing agent (compare with B) indicates that the highly reactive quinoid intermediates and products are responsible for the observed differences. (B) Difference spectra of the hydroxylation reaction (indicated by an increasing and narrow absorption band at 415 nm) (15, 16) catalyzed by AUS1 (Top) and abTYR (Bottom) in the presence of 1 mM sodium ascorbate as the reducing agent to trap the highly reactive quinoid intermediates and products. (C) Difference spectra of the hydroxylation reaction of kaempferol catalyzed by AUS1 in the presence of 5 mM H2O2 (41).