Fig. S9.
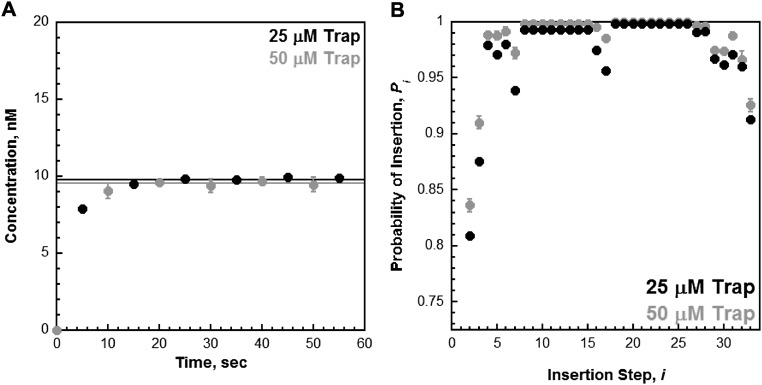
29ddC/62 acts as a passive trap. Primer extension was monitored as depicted in Fig. 2 with 250 µM of each dNTP, 0.5 mM ATP, and 25 µM 29ddC/62 trap. (A) Primer-extension products (total, ●) for holoenzyme formed with PCNA. For comparison, the data (black) are overlaid on data from Fig. S4A obtained under the same conditions except with 50 µM 29ddC/62 trap (gray). The primer extension products build up to the same level and then plateau thereafter, demonstrating that only a single turnover of primer extension is observed in the presence of the 29ddC/62 trap. (B) Probability of insertion (Pi, ●) for holoenzyme formed with PCNA. For comparison, the data (black) are overlaid on data from Fig. S6C obtained under the same conditions except with 50 µM 29ddC/62 trap (gray). Decreasing the concentration of 29ddC/62 trap twofold does not increase the probability of insertion (Pi) for any insertion step, in particular those for which Pi is low. For instance, Pi = 0.836 ± 0.00579 for i = 2 at 50 μM trap indicating that kpol is 5.11-fold greater than koff. If trapping was active, i.e., a second-order kinetic process, the observed rate constant for dissociation should decrease twofold when the concentration of trap is decreased twofold. In this scenario, kpol would now be 10.22-fold greater than koff and Pi should increase to 0.911. However, Pi remains constant for i = 2 at 25 μM trap (0.810 ± 0.0126). Together with the data presented in A, this demonstrates that 29ddC/62 trap does not actively assist in the dissociation of holoenzymes from the P29/Bio62 substrate. Rather, 29ddC/62 serves as a passive trap.