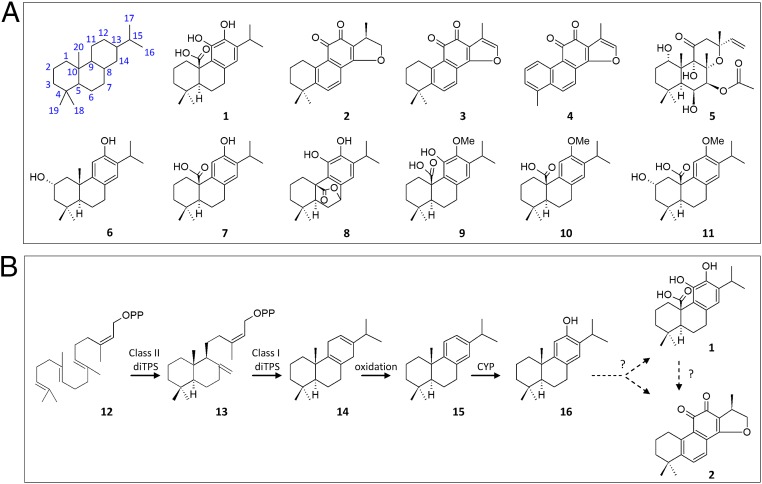
Fig. 1.
Chemical structures and biosynthesis of labdane-type diterpenes. (A) Chemical structures of the bioactive labdane-related diterpenes carnosic acid (1), cryptotanshinone (2), tanshinone IIA (3), tanshinone I (4), and forskolin (5) (Top). The main diterpenes isolated from S. pomifera leaves include salviol (6), pisiferic acid (7), carnosol (8), 12-methoxy-carnosic acid (9), O-methyl-pisiferic acid (10), and 2α-hydroxy-O-methyl-pisiferic acid (11) (Bottom). (B) The biosynthesis of tanshinone and carnosic acid begins with the cyclization of GGPP (12) by a class II diTPS to produce (+) copalyl diphosphate (CPP) (13), which is in turn converted to miltiradiene (14) by a class I diTPS. Spontaneous oxidation of 14 gives rise to abietatriene (15), which is oxidized to ferruginol (16) by a CYP enzyme. However, uncharacterized subsequent events lead to carnosic acid (1) and tanshinones (2–4).