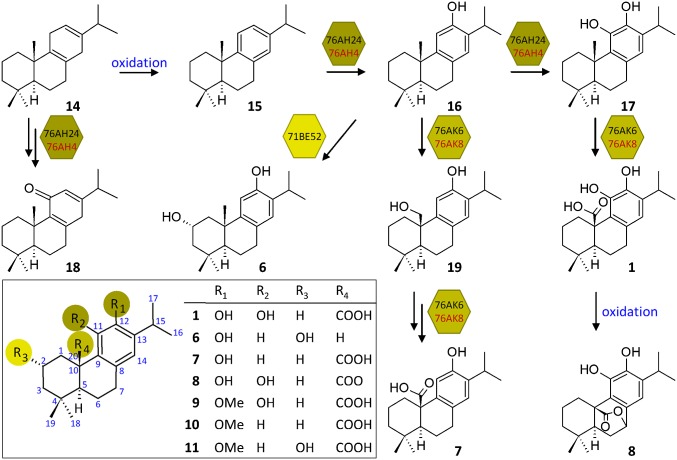
Fig. 5.
Proposed mechanism for the biosynthesis of carnosic-acid–related diterpenes in S. pomifera and R. officinalis. The biosynthesis of labdane-type diterpenes in S. pomifera (black) and R. officinalis (red) is initiated by the action of bifunctional enzymes, CYP76AH24 or CYP76AH4, respectively, which are responsible for the hydroxylation of 15 initially at position C-12 to produce 16 and subsequently at position C-11 to yield 17. The same bifunctional enzymes can also catalyze two successive oxidation events on 14 to yield 18. A S. pomifera enzyme, CYP71BE52, catalyzes oxidation of 16 at position 2α to synthesize 6. CYP76AK6 or CYP76AK8 catalyze successive oxygenations at position C-20 of 16 and 17 to yield 7 via 19, and 1, respectively. In the yeast platform, 1 is further oxidized to 8 by a yet undefined mechanism. The combined activities of CYP76AH24, CYP71BE52, and CYP76AK6 on the abietatriene skeleton (Inset) are sufficient to explain the biosynthesis of the main diterpenes (1, 6-11) isolated from S. pomifera and R. officinalis.