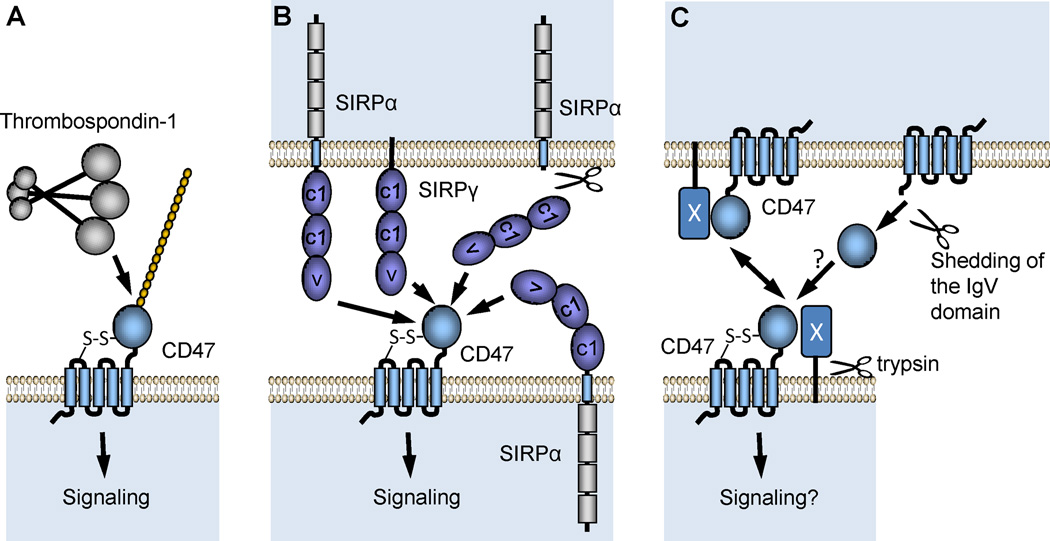
Fig. 1.
Topological arrangements of CD47 and its known extracellular ligands. (A) Thrombospondin-1 (TSP1) is a secreted protein that binds to a proteoglycan isoform of CD47 on the plasma membrane. Mutagenesis studies established that heparan sulfate modification of CD47 is required for high affinity interaction of TSP1 with CD47. (B) Two members of the signal recognition protein family (SIRPα and SIRPγ) bind to CD47 in a species-specific manner. These serve as counter-receptors for CD47 during cell-cell interactions, although evidence in smooth muscle cells suggests SIRPα can also interact in-cis with CD47 (Maile and Clemmons, 2003). Proteolytic cleavage of the extracellular domain of SIRPα can also generate a soluble ligand for CD47 (Umemori and Sanes, 2008). (C). Homotypic binding of CD47 to CD47 or binding to an unidentified trypsin-sensitive counter-receptor (X) can mediate cell-cell interactions (Rebres et al., 2005). The IgV domain of CD47 can be shed under some conditions (Maile et al., 2008b, Kaur et al., 2011), but the ability of this domain to engage in homotypic binding as a soluble CD47 ligand is not known.