Fig. 2.
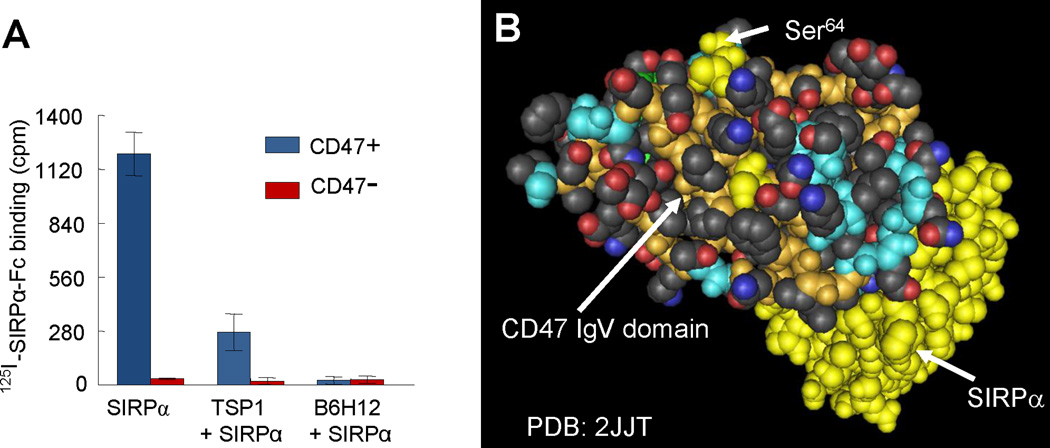
Evidence for the relationship between TSP1 and SIRPα binding sites on CD47. (A) Data from Isenberg et al (Isenberg et al., 2009a) demonstrates that TSP1 and the function-blocking CD47 antibody B6H12 inhibit binding of the radiolabeled extracellular domain of SIRPα to cells expressing CD47. Lack of binding to an isogenic cell line lacking CD47 confirms specificity of SIRPα binding. These competition data could indicate that TSP1 and SIRPα bind to overlapping sites on CD47 or that binding of these proteins to distinct sites on CD47 results in steric or allosteric inhibition of SIRPα binding by TSP1. (B) A space-filling projection of the crystal structure of the extracellular IgV domain of human CD47 (tan backbone with colored side chains) complexed with the terminal Ig domain of SIRPα (yellow). Mutagenesis established that post-translational modification of Ser64 on CD47 (yellow) is required for TSP1 signaling. This suggests that the TSP1 binding site is distinct from the SIRPα binding site on CD47.