Fig. 3.
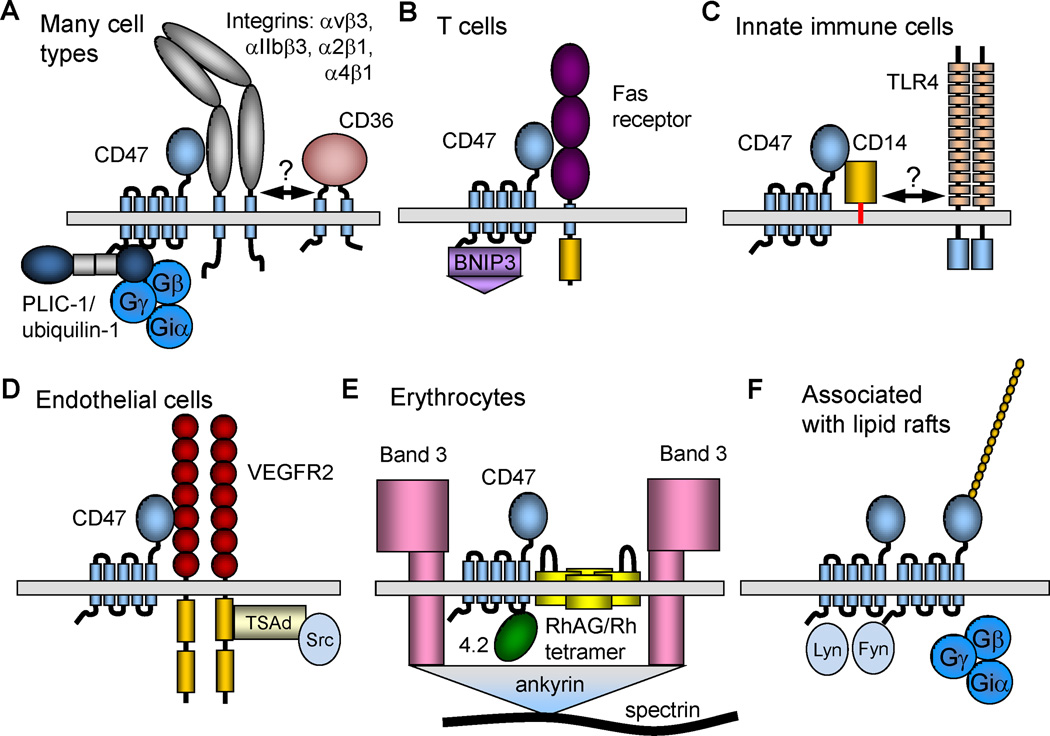
Supramolecular complexes containing CD47. (A) On a majority of cell types CD47 laterally associates with specific integrins. These integrins in turn may associate with other membrane proteins, potentially including CD36 in neuronal cells (Bamberger et al., 2003). However, readers are cautioned that the proposed CD36-α6β1-CD47 complex has not been directly demonstrated. PLIC1/ubiquilin-1 directly binds to the C-terminal cytoplasmic tail of CD47 and can recruit heterotrimeric G-proteins to CD47. (B) In T cells CD47 can laterally associate with Fas receptor. Yeast two-hybrid screening identified BNIP3 as another direct binding partner for the C-terminal tail of CD47. (C) In innate immune cells FRET studies identified lateral association of CD47 with the LPS co-receptor CD14. CD14 is also a component of the TLR4 signaling complex, but it is unclear whether CD447 can bind to CD14 while it is in the TLR complex. (D) In endothelial cells CD47 closely associates with VEGFR2. This complex also contains Src kinases, which associate with the cytoplasmic tail of VEGFR2 via TSAd (Shibuya, 2006). (E) Human erythrocytes lack integrins, and CD47 is found in a multiprotein complex that accounts for Rh-antigen activity. This complex also contains Band 3 and is anchored to the red cell cytoskeleton via ankyrin. Band 4.2 is required for maintaining CD47 in this complex. (F) Lateral oligomerization of CD47 has been reported in association with lipid rafts. Heterotrimeric G protein and the Src kinases Lyn and Fyn are enriched in this complex.