Abstract
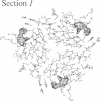
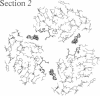
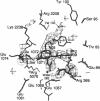
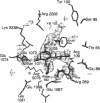
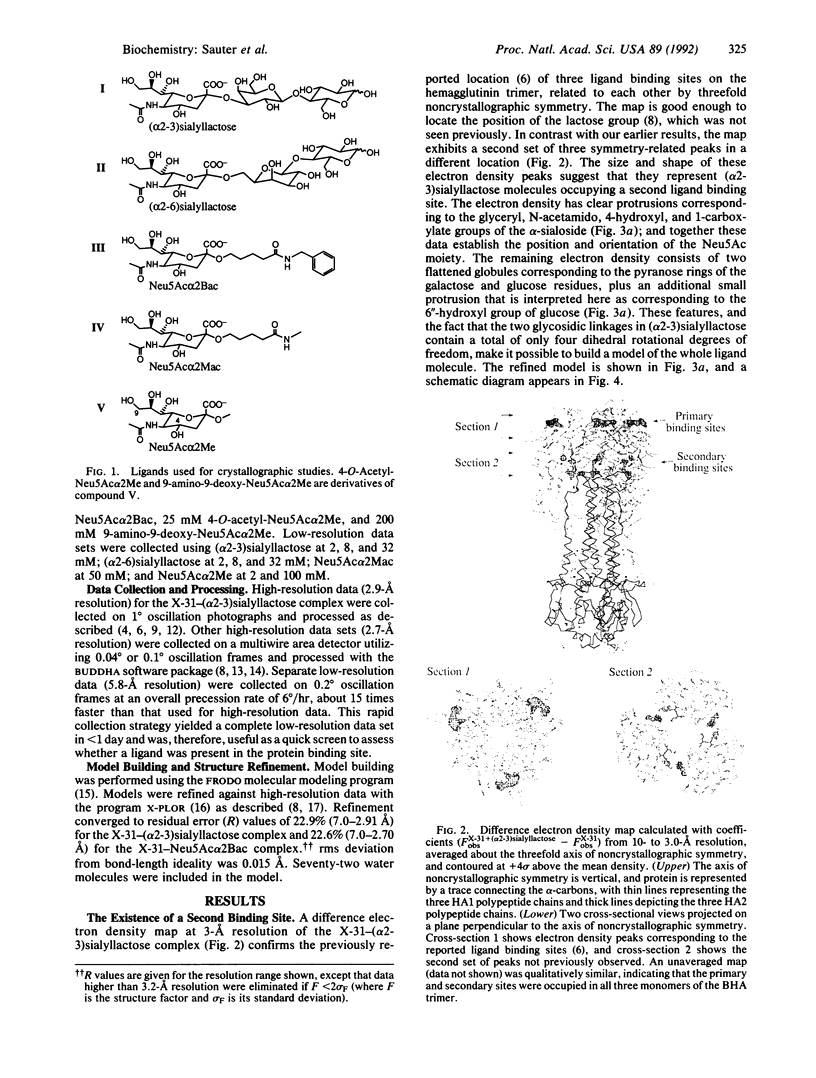
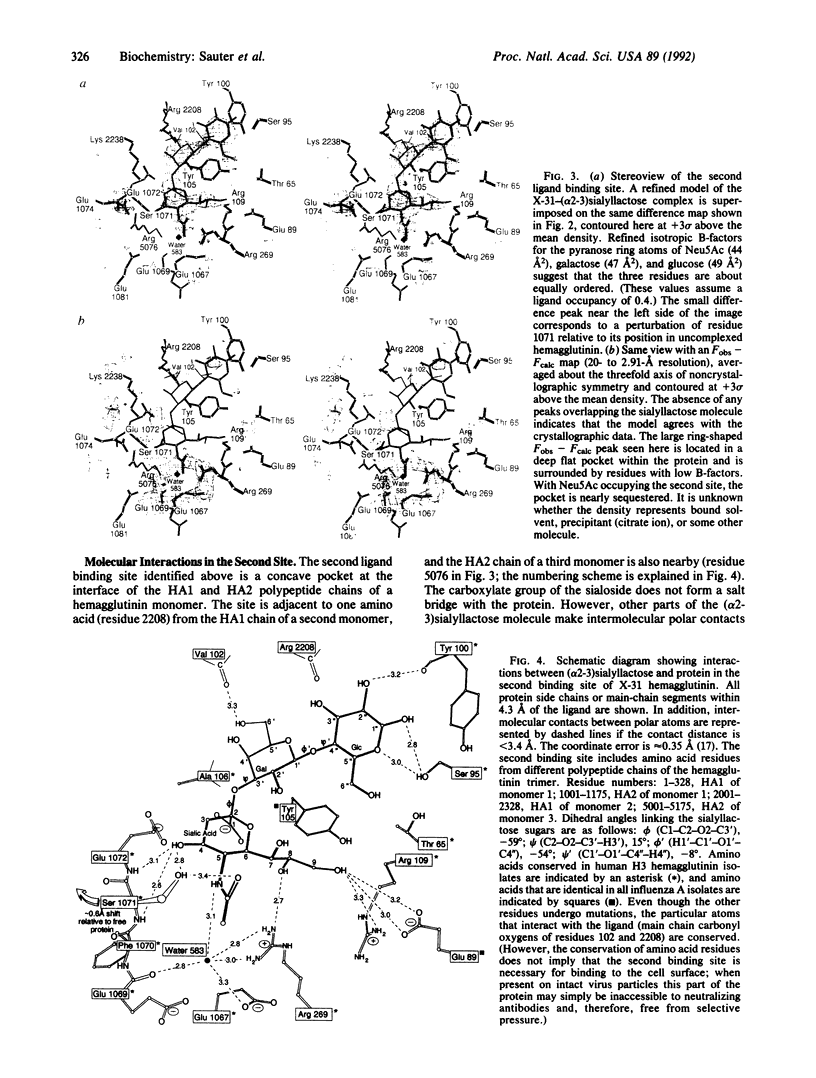
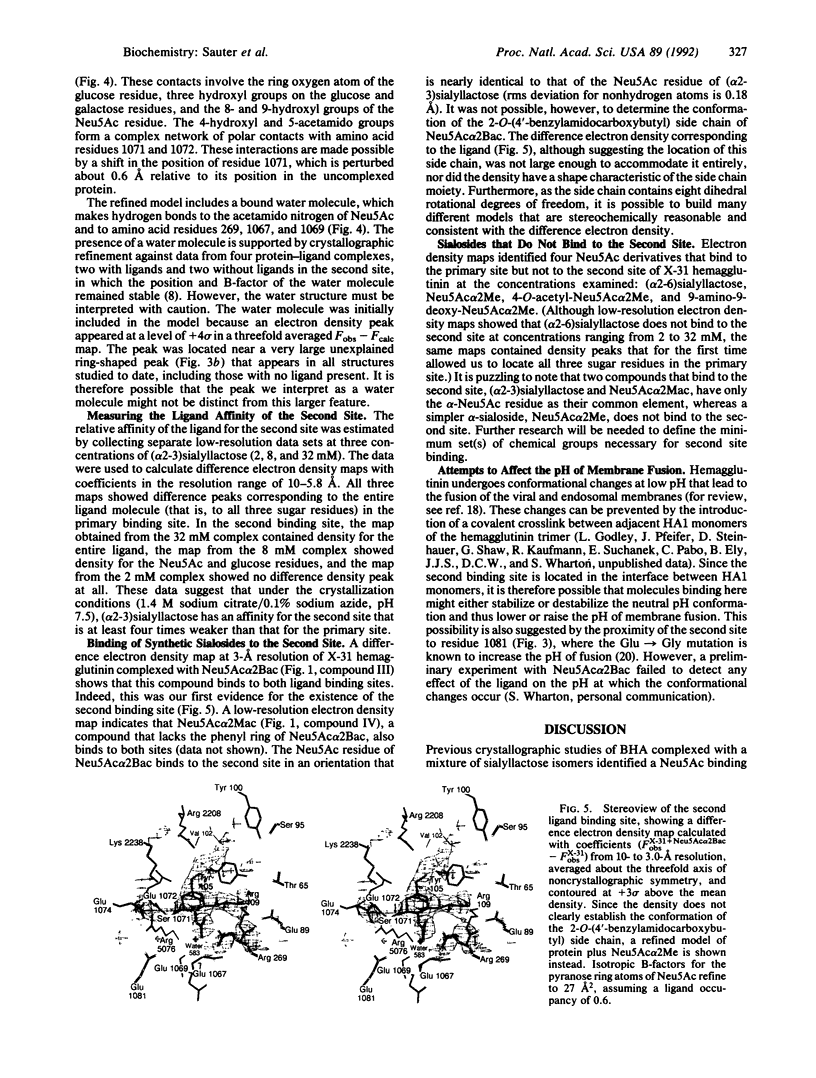
X-ray crystal structures have been determined for several complexes between influenza virus hemagglutinin and derivatives of its cell-surface receptor, sialic acid (Neu5Ac). Difference electron density maps establish the existence of a second binding site in addition to the primary site characterized previously. Three compounds bind to both sites: Neu5Ac(alpha 2-3)Gal(beta 1-4)Glc [(alpha 2-3)sialyllactose], alpha-2-O-(4'-benzylamidocarboxybutyl)-5-N-acetylneuraminic acid, and alpha-2-O-(4'-methylamidocarboxybutyl)-5-N-acetylneuraminic acid; and four other compounds bind only to the primary site: Neu5Ac(alpha 2-6)Gal(beta 1-4)Glc [(alpha 2-6)sialyllactose], alpha-2-O-methyl-5-N-acetylneuraminic acid, 4-]-acetyl-alpha-2-O-methyl-5-N-acetylneuraminic acid, and 9-amino-9-deoxy-alpha-2-O-methyl-5-N-acetylneuraminic acid. The maps also extend earlier results by showing the location of all three sugar residues of (alpha 2-3)sialyllactose in the primary binding site. The affinity of (alpha 2-3)sialyllactose for the second site was estimated by collecting x-ray diffraction data at various ligand concentrations and was found to be at least four times weaker than its affinity for the primary site. Although it is not yet known whether the second binding site participates in the infection process, it nevertheless offers a potential target for the design of antiviral drugs.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergh M. L., Koppen P., van den Eijnden D. H. High-pressure liquid chromatography of sialic acid-containing oligosaccharides. Carbohydr Res. 1981 Aug 1;94(2):225–229. doi: 10.1016/s0008-6215(00)80720-x. [DOI] [PubMed] [Google Scholar]
- Brand C. M., Skehel J. J. Crystalline antigen from the influenza virus envelope. Nat New Biol. 1972 Aug 2;238(83):145–147. doi: 10.1038/newbio238145a0. [DOI] [PubMed] [Google Scholar]
- Daniels R. S., Downie J. C., Hay A. J., Knossow M., Skehel J. J., Wang M. L., Wiley D. C. Fusion mutants of the influenza virus hemagglutinin glycoprotein. Cell. 1985 Feb;40(2):431–439. doi: 10.1016/0092-8674(85)90157-6. [DOI] [PubMed] [Google Scholar]
- Durbin R. M., Burns R., Moulai J., Metcalf P., Freymann D., Blum M., Anderson J. E., Harrison S. C., Wiley D. C. Protein, DNA, and virus crystallography with a focused imaging proportional counter. Science. 1986 May 30;232(4754):1127–1132. doi: 10.1126/science.3704639. [DOI] [PubMed] [Google Scholar]
- Jones T. A. Diffraction methods for biological macromolecules. Interactive computer graphics: FRODO. Methods Enzymol. 1985;115:157–171. doi: 10.1016/0076-6879(85)15014-7. [DOI] [PubMed] [Google Scholar]
- Knossow M., Daniels R. S., Douglas A. R., Skehel J. J., Wiley D. C. Three-dimensional structure of an antigenic mutant of the influenza virus haemagglutinin. Nature. 1984 Oct 18;311(5987):678–680. doi: 10.1038/311678a0. [DOI] [PubMed] [Google Scholar]
- Matrosovich M. N., Mochalova L. V., Marinina V. P., Byramova N. E., Bovin N. V. Synthetic polymeric sialoside inhibitors of influenza virus receptor-binding activity. FEBS Lett. 1990 Oct 15;272(1-2):209–212. doi: 10.1016/0014-5793(90)80486-3. [DOI] [PubMed] [Google Scholar]
- Perkins S. J., Johnson L. N., Phillips D. C., Dwek R. A. The binding of monosaccharide inhibitors to hen egg-white lysozyme by proton magnetic resonance at 270 MHz and analysis by ring-current calculations. Biochem J. 1981 Feb 1;193(2):553–572. doi: 10.1042/bj1930553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchett T. J., Paulson J. C. Basis for the potent inhibition of influenza virus infection by equine and guinea pig alpha 2-macroglobulin. J Biol Chem. 1989 Jun 15;264(17):9850–9858. [PubMed] [Google Scholar]
- Rogers G. N., Paulson J. C., Daniels R. S., Skehel J. J., Wilson I. A., Wiley D. C. Single amino acid substitutions in influenza haemagglutinin change receptor binding specificity. Nature. 1983 Jul 7;304(5921):76–78. doi: 10.1038/304076a0. [DOI] [PubMed] [Google Scholar]
- Sauter N. K., Bednarski M. D., Wurzburg B. A., Hanson J. E., Whitesides G. M., Skehel J. J., Wiley D. C. Hemagglutinins from two influenza virus variants bind to sialic acid derivatives with millimolar dissociation constants: a 500-MHz proton nuclear magnetic resonance study. Biochemistry. 1989 Oct 17;28(21):8388–8396. doi: 10.1021/bi00447a018. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Nagao Y., Kato H., Matsumoto M., Nerome K., Nakajima K., Nobusawa E. Human influenza A virus hemagglutinin distinguishes sialyloligosaccharides in membrane-associated gangliosides as its receptor which mediates the adsorption and fusion processes of virus infection. Specificity for oligosaccharides and sialic acids and the sequence to which sialic acid is attached. J Biol Chem. 1986 Dec 25;261(36):17057–17061. [PubMed] [Google Scholar]
- Toogood P. L., Galliker P. K., Glick G. D., Knowles J. R. Monovalent sialosides that bind tightly to influenza A virus. J Med Chem. 1991 Oct;34(10):3138–3140. doi: 10.1021/jm00114a025. [DOI] [PubMed] [Google Scholar]
- Weis W. I., Brünger A. T., Skehel J. J., Wiley D. C. Refinement of the influenza virus hemagglutinin by simulated annealing. J Mol Biol. 1990 Apr 20;212(4):737–761. doi: 10.1016/0022-2836(90)90234-D. [DOI] [PubMed] [Google Scholar]
- Weis W., Brown J. H., Cusack S., Paulson J. C., Skehel J. J., Wiley D. C. Structure of the influenza virus haemagglutinin complexed with its receptor, sialic acid. Nature. 1988 Jun 2;333(6172):426–431. doi: 10.1038/333426a0. [DOI] [PubMed] [Google Scholar]
- Wiley D. C., Skehel J. J. Crystallization and x-ray diffraction studies on the haemagglutinin glycoprotein from the membrane of influenza virus. J Mol Biol. 1977 May 15;112(2):343–347. doi: 10.1016/s0022-2836(77)80149-6. [DOI] [PubMed] [Google Scholar]
- Wiley D. C., Skehel J. J. The structure and function of the hemagglutinin membrane glycoprotein of influenza virus. Annu Rev Biochem. 1987;56:365–394. doi: 10.1146/annurev.bi.56.070187.002053. [DOI] [PubMed] [Google Scholar]
- Wilson I. A., Skehel J. J., Wiley D. C. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature. 1981 Jan 29;289(5796):366–373. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]