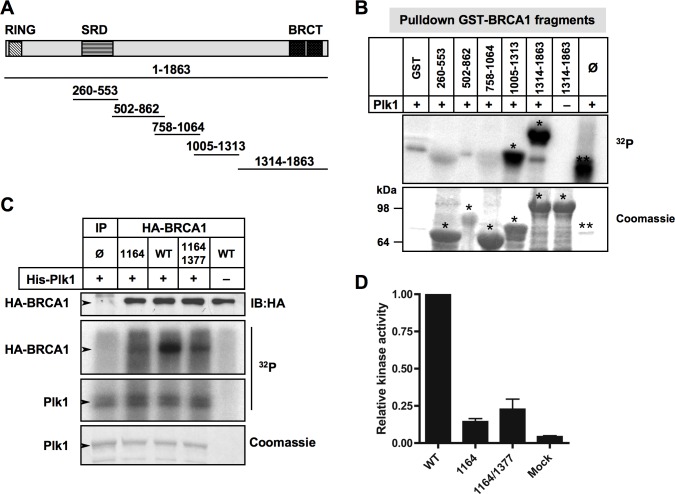
Figure 5. Plk1 phosphorylates BRCA1 mainly at Ser1164 residue in vitro.
A. Schematic illustration of GST-tagged BRCA1 fragments used as substrates in kinase reaction mixtures described in (B). B. Recombinant Plk1 protein phosphorylates GST-BRCA1 fragments aa1005-1313 and aa 1314-1863. His-Plk1 was incubated with purified GST-BRCA1 fragments and [γ32P]ATP to determine the Plk1-mediated phosphorylation. The asterisks in top panel (32P) mark GST-BRCA1 fragments that were phosphorylated with Plk1 (*) and Plk1 autophosphorylation (**). No signal was detected with GST alone or in absence of Plk1, indicating the specificity of the Plk1 kinase reaction. The SDS-PAGE gel stained by Coomassie blue illustrates the amount of purified GST-BRCA1 fragments used in the kinase reaction. The asterisks in bottom panel (Coomassie) indicate purified GST-BRCA1 fragments (*) and recombinant Plk1 (**). Φ, no addition of GST fragment. C. Recombinant Plk1 protein phosphorylates HA-tagged BRCA1 mainly on S1164. HeLa cells were transfected with plasmids encoding HA-tagged wild-type BRCA1 (WT), or mutated HA-tagged BRCA1 (S1164C, S1164C/S1377C). 24 h following transfection, HA-tagged BRCA1 proteins were immunoprecipitated and either subjected to immunoblotting or used in a kinase assay. Equivalent expression of the tagged proteins was confirmed by immunoblotting (IB) using anti-HA antibody. Immunoprecipitated HA-tagged BRCA1 proteins were incubated with recombinant Plk1 protein in the presence of [γ32P]ATP. The reaction mixtures were resolved by SDS-PAGE and visualized first by Coomassie blue staining and then with a FLA-3000 scanner. No [γ32P] signal was detected using control IP or without addition of Plk1 protein, showing the specificity of the Plk1 kinase reaction. Note that Plk1 phosphorylates itself. IP Φ, control IP. D. Histogram represents the relative phosphorylation of various immunoprecipitated HA-BRCA1 proteins by Plk1. [γ32P] signal detected via FLA-3000 Imager scans of the dried gels was quantified and presented as relative to level of [γ32P] incorporated in HA-wtBRCA1. Mock, control IP.