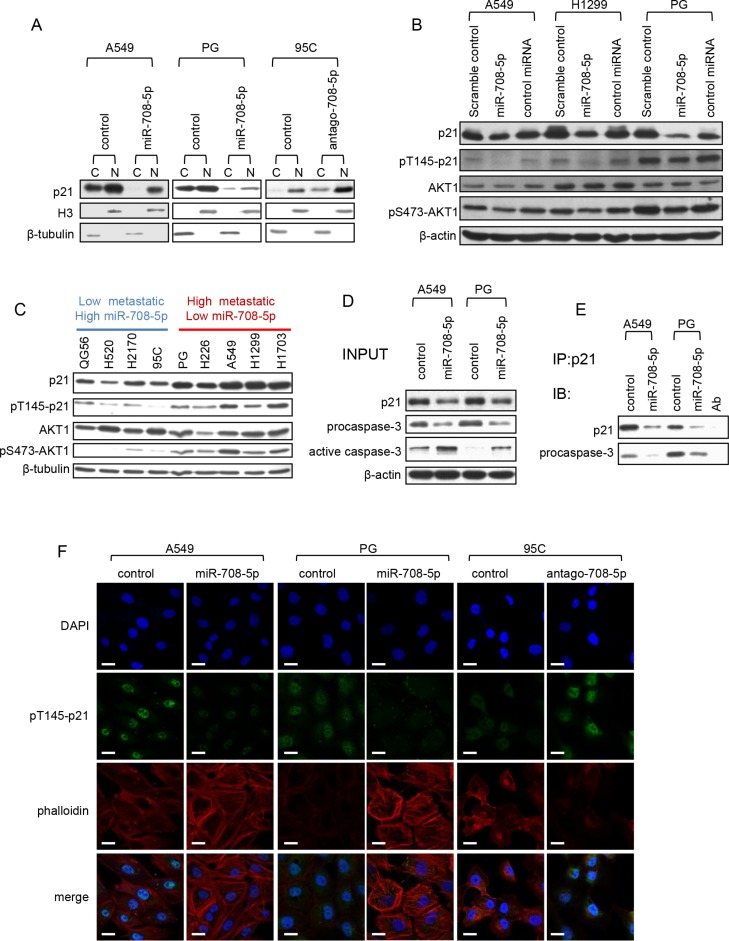
Figure 4. miR-708-5p inhibits cytoplasmic localization of p21.
(A) Subcellular fractionation assay was carried out to determine the cellular localization of p21 in the A549 and PG cells transfected with miR-708-5p and control mimics, and 95C cells transfected with antago-708-5p and antago-control. Nuclear and cytoplasmic fractions were respectively subjected to immunoblotting analysis with antibody against p21. Histone 3 (H3) and β-tubulin were used as loading controls for nuclear and cytoplasmic proteins respectively. (B) Immunoblotting of p21, pT145-p21, AKT1 and pS473-AKT1 in the A549, H1299 and PG cells transfected with the miR-708-5p mimics, a control miRNA mimics (miR-708-3p) or scramble control mimics. β-actin was used as a loading control. (C) Immunoblotting of p21, pT145-p21, AKT1 and pS473-AKT1 in QG56, H520, H2170, 95C, PG, H226, A549, H1299 and H1703 cell lines. (D) Co-immunoprecipitation of endogenous p21 and detection of endogenous procaspase-3. The A549 and PG cells, which were serum deprived for 48 h, were stimulated by exposure to 10% serum for 6 h. A portion (5%) of the whole cell lysates was used as input for the immunoblotting analysis, and β-actin was used as a loading control. (E) Endogenous p21 was immunoprecipitated from 2 mg of the protein of A549 and PG cell with the p21 antibody, and probed with p21 and procaspase-3 antibodies in the western blotting assay. (F) Confocal images of the A549 and PG cells transfected with the miR-708-5p or control mimics for localization of pT145-p21 and cytoskeleton, and 95C cells transfected with antago-708-5p or control. The cells were synchronized after serum deprivation for 48 h and then re-stimulated with 10% serum for 6 h. Scale bars, 30 μm.