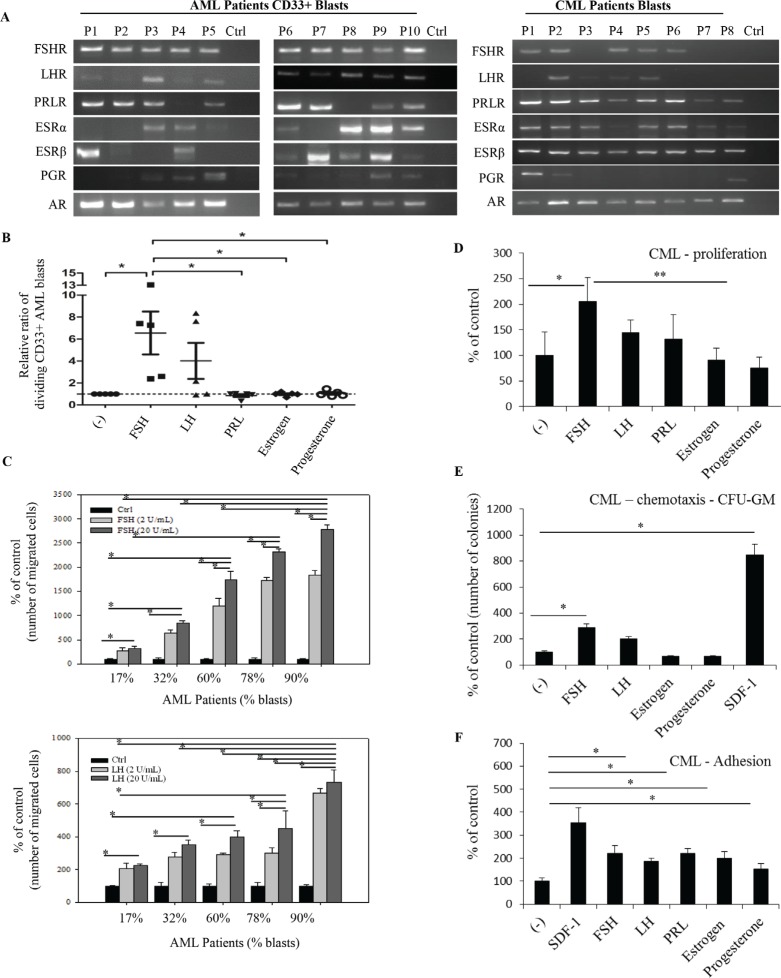
Figure 5. Functional SexH receptors are expressed by CD33+ AML and CML primary human patient blasts.
(A) Expression of SexH (pituitary and gonadal) receptor transcripts was detected in purified mRNA samples from CD33+ blasts sorted from AML human patients (n = 10) and CML human patient blasts (n = 8) by reverse transcription polymerase chain reaction (RT-PCR). Samples with water only instead of cDNA were used as negative controls. Representative agarose gels of the RT-PCR amplicons obtained are shown. (B) The effect of pituitary and gonadal SexHs on proliferation of CD33+AML blasts. The ratio of the number of dividing CD33+ AML blasts (that diluted CFSE) following stimulation with SexHs is normalized to unstimulated spontaneous blast division (dotted line). Repeated measures ANOVA test with post hoc Bonferroni multiple comparison test was used. Lines represent mean values, while whiskers represent standard error of the mean (SEM), and *p ≤ .05 is considered significant. (C) The effect of pituitary SexHs (FSH and LH) on transmigration of other primary mononuclear cells isolated from peripheral blood of AML patients (n = 8) with variable blast percentages. (D) Proliferation of peripheral blood mononuclear leukemia cells isolated from CML patients in response to SexHs. *P ≤ .04 and **p ≤ .03 are considered significant. (E) CFU-GM activity of leukemic cells isolated from primary CML patients that transmigrated through Transwell membranes in response to pituitary and gonadal SexHs. *p ≤ .05 versus control. (F) Pituitary and gonadal hormones stimulate the fibronectin adhesiveness of leukemia cells isolated from CML patients. These cells (2.5 × 103/100 μL/well) were made quiescent and afterwards stimulated with SexHs at the indicated concentrations in RPMI medium plus 0.5% BSA for 5 min incubation. After the non-adherent cells were removed by washing three times with PBS, the number of adherent cells was then scored. The negative control values are normalized to 100%. Data from two separate experiments are merged together (each experiment has been performed in duplicate) and means ± SD are shown. *p ≤ .05 versus the control is considered significant. Stromal-derived factor 1 and 0.5% BSA in RPMI medium were used as a positive and a negative control, respectively.