Figure 4. OLA1 inhibits PP1 reactivation by blocking GSK3β from phosphorylation of I-2.
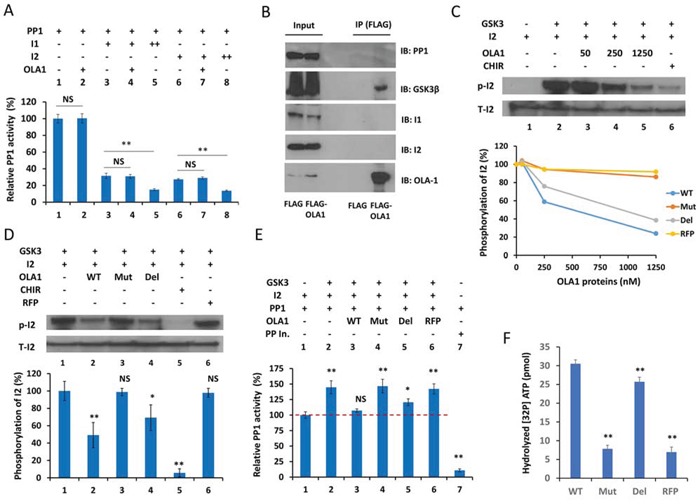
A. OLA1 does not directly inhibit PP1 activity or have an effect on the Inhibitor 1- or Inhibitor 2-mediated inhibition of PP1 (I-1 +: 10 ng/μl, ++: 20 ng/μl; I-2 +: 20 ng/μl, ++: 40 ng/μl; OLA1 +: 250 nM; incubation time: 30 min). PP1 activity was measured with the DiFMUP reagent. B. Co-IP of OLA1 with GSK3β but not with PP1, I-1, or I-2. C. OLA1 inhibits GSK3 activity in an in vitro I-2 phosphorylation assay. Top: immunoblot analysis shows that recombinant OLA1 WT protein decreases I-2 phosphorylation in a dose-response manner. The GSK3-specific inhibitor CHIR (2 μM) was used as the positive control. Bottom: dose-response curves of I-2 phosphorylation inhibition by OLA1 WT, N230A (Mut), and deltaTGS (Del) proteins, with RFP used as negative control. D. Quantitative analysis of I-2 phosphorylation inhibition by OLA1 WT and mutant proteins (250 nM, 1 hour). CHIR (20 μM) was used as a positive control and RFP was used as a negative control. E. OLA1 inhibits the GSK3β-mediated reactivation of PP1. GSK3β was added to the previously formed PP1 · I-2 complex, in which PP1 was inactivated to a basal level (designated here as 100%). Co-addition of OLA1 WT and Del proteins results in a total and partial blockage of the PP1 reactivation, respectively, whereas Mut and RFP proteins show no effect. All proteins were used at 250 nM for 1 hour. Protein phosphatase inhibitor (Roche) was used as a positive control. F. ATPase activity of OLA1 proteins. A 32P-γ-ATP-based assay was used to measure the ATP hydrolyzing activity of OLA1 WT and mutant proteins. RFP was used as a negative control. Data in all bar graphs are presented as means ± SD. *p < 0.05; **p < 0.01 (as compared with line 1 of each graph or between the bars as indicated; Student's t-test).
