Fig. 3.
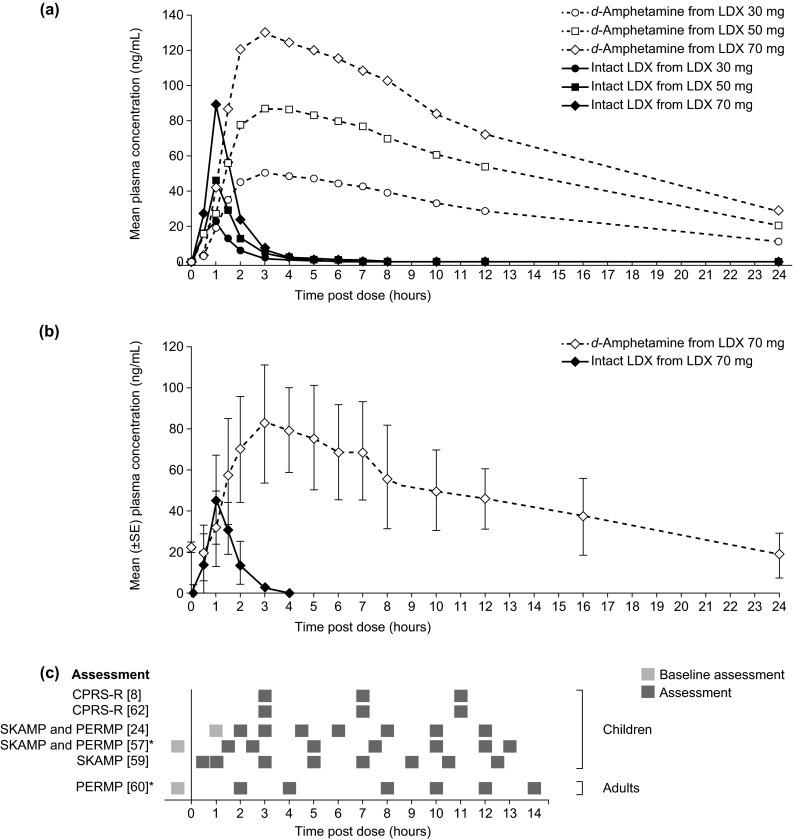
Pharmacokinetic profiles of plasma LDX and d-amphetamine a after a single oral dose of LDX in children with ADHD (N = 17) [14] (Reproduced from ‘Pharmacokinetics of lisdexamfetamine dimesylate and its active metabolite, d-amphetamine, with increasing oral doses of lisdexamfetamine dimesylate in children with attention-deficit/hyperactivity disorder: a single-dose, randomized, open-label, crossover study’, Boellner SW et al. Clinical Therapeutics 2010;32:252–64. ©2010 Excerpta Medica Inc. Reproduced with permission from Elsevier), b on day 7 of daily oral LDX dosing in healthy adults (N = 11) [15] (Krishnan SM and Stark JG, Current Medical Research and Opinion 2008;24:33–40, copyright ©2008 Informa Healthcare. Adapted with permission of Informa Healthcare), and c timings of symptomatological (SKAMP, CPRS-R) or functional (PERMP) assessments in studies of the efficacy of LDX throughout the day [8, 24, 57, 59, 60, 62]. Asterisk Effect sizes for LDX versus placebo in these studies are presented in Fig. 4. Biederman et al. [24] used a post-dose time point as baseline. ADHD attention-deficit/hyperactivity disorder, CPRS-R Connors’ Parent Rating Scale-Revised, LDX lisdexamfetamine dimesylate, PERMP Permanent Product Measure of Performance, SKAMP Swanson, Kotkin, Agler, M-Flynn, and Pelham
