Figure 7.
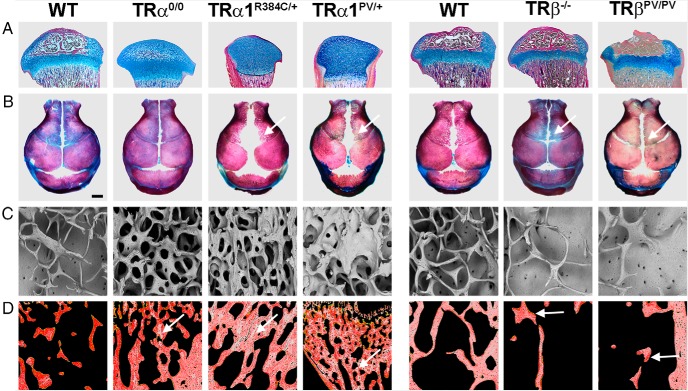
Skeletal phenotype of TRα and TRβ mutant mice. A, Proximal tibias stained with alcian blue (cartilage) and van Gieson (bone, red) showing delayed formation of the secondary ossification center in TRα-deficient mice (TRα0/0) and grossly delayed formation in mice with dominant-negative TRα mutations (TRα1R384C/+ and TRα1PV/+). Mice with mutation or deletion of TRβ have advanced ossification with premature growth plate narrowing. B, Skull vaults stained with alizarin red (bone) and alcian blue (cartilage) showing skull sutures and fontanelles. Arrows indicate delayed intramembranous ossification in mice with dominant-negative TRα mutations (TRα1R384C/+ and TRα1PV/+) and advanced ossification in mice with mutation or deletion of TRβ. C, Trabecular bone microarchitecture in adult TR mutant mice. Backscattered electron scanning electron microscopy images show increased trabecular bone in TRα0/0 mice and severe osteosclerosis in TRα1R384C/+ and TRα1PV/+ mice. By contrast, TRβ mutant mice have reduced trabecular bone volume and osteoporosis. D, Trabecular bone micromineralization in adult TR mutant mice. Pseudo-colored quantitative backscattered electron scanning electron microscopy images showing mineralization densities in which high mineralization density is gray and low density is red. Mice with deletion or mutation of TRα have retention of highly mineralized calcified cartilage (arrows) demonstrating a persistent remodeling defect. By contrast, mice with deletion or mutation of TRβ have reduced bone mineralization (arrow) secondary to increased bone turnover.